Introduction
In the dynamic landscape of medical device regulation, post-market clinical follow-up (PMCF) studies stand as a crucial practice to confirm the safety and efficacy of medical devices after they have been released into the market. These studies bridge the gap between pre-market testing and long-term performance, specifically in diverse real-world patient populations that may not have been fully represented in clinical trials.
PMCF studies are instrumental in monitoring outcomes, identifying adverse events, and optimizing device usage, amid rapidly advancing technological innovations. As medical devices progress in complexity, especially those that are central to patient survival or involve implantable technology, the regulatory environment intensifies correspondingly.
According to estimates, high-risk devices, such as those that are implantable and sustain or support life, make up about 10% of devices regulated by the FDA but are subjected to stringent scrutiny. The comprehensive data collected through PMCF studies are vital, capturing details regarding the device type, manufacturer, brand, and lot number, along with the nature of any device-related problems. The vigilant reporting of any device malfunctions, defects, or adverse events experienced during patient use is indispensable for maintaining a robust safety profile. These activities ensure that as the device landscape evolves and patient treatment options become more sophisticated, the efficacy and safety of medical devices in Latin America are upheld, benefiting patients and healthcare systems alike.
Understanding Post-Market Clinical Follow-Up Studies
In the dynamic landscape of medical device regulation, post-market clinical follow-up (PMCF) studies stand as a crucial practice to confirm the safety and efficacy of medical devices after they have been released into the market. These studies bridge the gap between pre-market testing and long-term performance, specifically in diverse real-world patient populations that may not have been fully represented in clinical trials.
PMCF studies are instrumental in monitoring outcomes, identifying adverse events, and optimizing device usage, amid rapidly advancing technological innovations. As medical devices progress in complexity, especially those that are central to patient survival or involve implantable technology, the regulatory environment intensifies correspondingly.
According to estimates, high-risk devices, such as those that are implantable and sustain or support life, make up about 10% of devices regulated by the FDA but are subjected to stringent scrutiny. The comprehensive data collected through PMCF studies are vital, capturing details regarding the device type, manufacturer, brand, and lot number, along with the nature of any device-related problems. The vigilant reporting of any device malfunctions, defects, or adverse events experienced during patient use is indispensable for maintaining a robust safety profile. These activities ensure that as the device landscape evolves and patient treatment options become more sophisticated, the efficacy and safety of medical devices in Latin America are upheld, benefiting patients and healthcare systems alike.
Importance of Post-Market Surveillance
The imperative for vigilance in post-market surveillance of medical devices is clear: it serves as the cornerstone for safeguarding patient safety by systematically tracking the performance of devices after they have been approved for the market. Acknowledging that controlled environments such as clinical trials may not uncover all potential issues, the real-world data that post-market surveillance collects is pivotal.
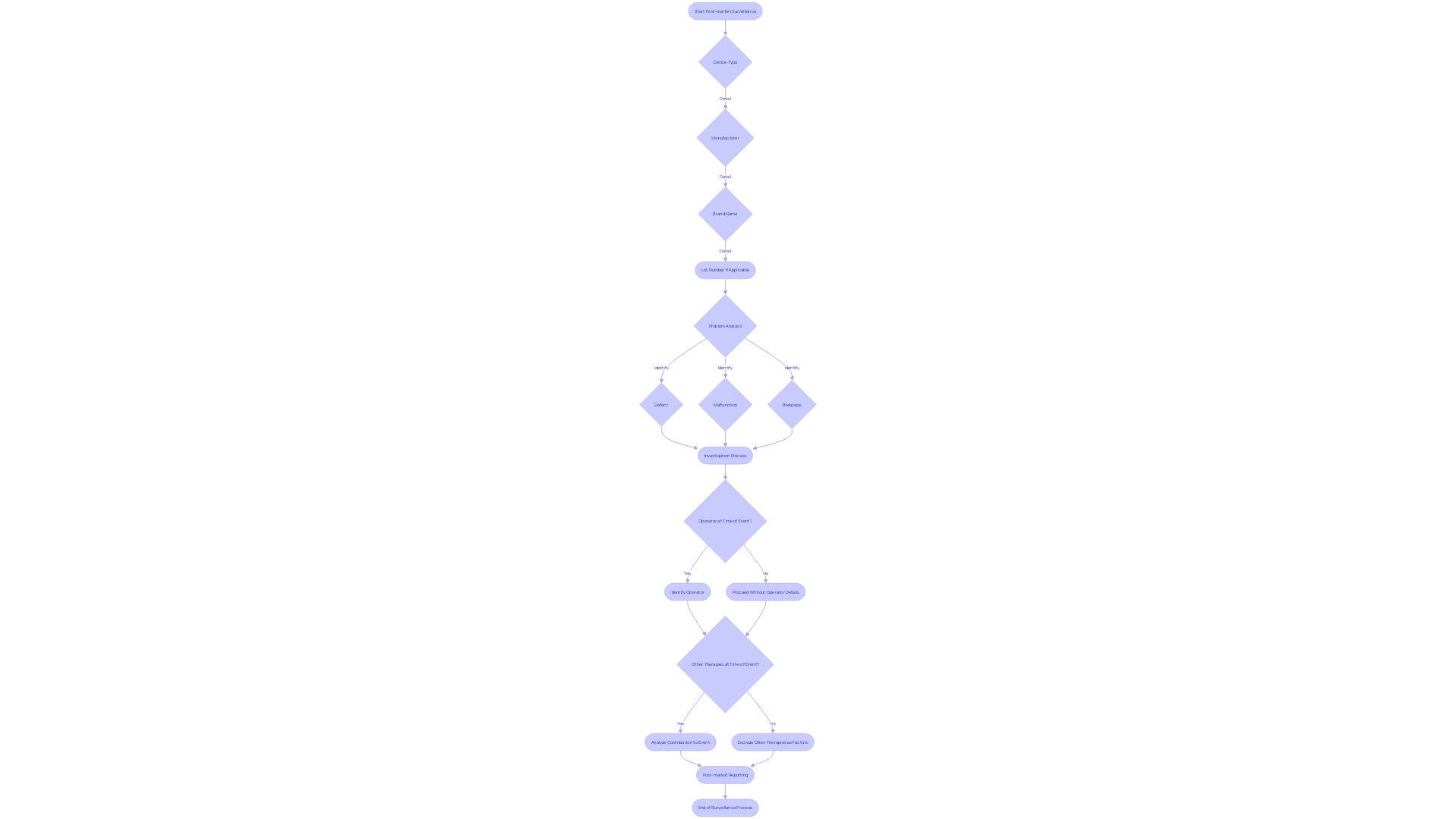
It delves into specific details such as the device type, manufacturer, brand name, and lot number, to recognize patterns or isolated incidents that signal a device defect, malfunction, or breakage. Such scrutiny is crucial when inquiring if any adverse events occurred while the device was in operation and who was operating it, along with considering other simultaneous therapies that could influence outcomes.
This granular approach to post-market reporting of adverse events, use errors, and product problems enhances transparency and drives much-needed modifications to device usage or design. As regulatory demands intensify globally, summits like the MedTech Regulatory Intelligence Summit underscore the evolving landscape, emphasizing the need for a proactive regulatory strategy that includes robust post-market surveillance systems. This vigilance is more than a regulatory obligation; it is a moral one, ensuring that every medical device continues to serve its intended role with assured safety and efficacy.
Regulatory Requirements for Post-Market Clinical Follow-Up Studies
In the wake of groundbreaking medical device innovations across Latin America, regulatory oversight has become paramount to ensure the safety and efficacy of these devices throughout their market life. Scrutiny does not cease at market approval; instead, vigilant post-market clinical follow-up (PMCF) studies are mandated to identify and mitigate any potential risks that could arise during regular use.
The meticulous reporting of adverse events, use errors, device malfunctions, and product problems form the cornerstone of such studies. Reporting protocols require detailed documentation that includes the device type, brand name, and lot number, as well as the context of the event, such as whether the device was in operation and concurrent therapies.
Persistent challenges confront those in the medical device industry, who must navigate an ever-evolving regulatory landscape, with agencies like the FDA emphasizing the need for rigorous development and validation of Digital Health Technologies (DHTs). The FDA's approach aligns with broader international regulatory expectations, requiring manufacturers to maintain stringent vigilance over their products post-market.
One such example includes the Digital Health Technologies for Remote Data Acquisition in Clinical Investigations, a framework designed to ensure the reliability of data captured remotely through medical devices. These rigorous standards serve dual purposes: they protect patient safety and affirm manufacturers' commitment to maintaining compliance with regulatory authorities. Therefore, understanding and adherence to these guidelines, which include tracking and examining adverse events in granularity, are critical. Elements to be tracked include device defects, operator identity, and potential contributory factors – all data contributing significantly to the safety net that surrounds medical devices after they enter the market.
Designing a Post-Market Clinical Follow-Up Study
Optimally executing post-market clinical follow-up studies is of paramount importance in the Latin American region, given its burgeoning medical device market. Customary design choices include prospective cohort studies, registry studies, or case-control studies—each offering unique advantages depending on the research objectives and resources at hand.
Ensuring patient safety and efficacy of these devices mandates meticulous planning from the outset, especially regarding the selection of the study population, the establishment of inclusion and exclusion criteria, and the employ of rigorous data collection methods. The duration of follow-up must be methodically considered, while statistical analyses require meticulous attention to ensure unwavering validity and the necessary statistical power to support conclusive outcomes.
In this nuanced landscape, ethical, legal, and social considerations assume unprecedented salience. The governance of technology within this space necessitates a deep understanding of the market incentives and intellectual property rights shaping technological trajectories—dimensions encapsulated by various case studies that concretize the ethical quandaries at play.
Reporting adverse events or product problems encompasses not only a thorough documentation of the device type, manufacturer, and lot number but also requires a probing inquiry into any concurrent therapies that may render causal attributions complex. This pursuit aligns with broader social goals aimed at improving healthcare outcomes, reflected in the earnest discussions at FDA public meetings on the use of Digital Health Technologies (DHTs) in clinical trials. The discourse around the validation and verification of DHTs, underscored by an FDA draft guidance, denotes the industry’s directed efforts toward achieving precision in remote data acquisition. At the heart of these discussions lies a stringent validation process, one that bears the weight of the data's integrity and, by extension, the welfare of the patient community—reiterating the imperative of a meticulous approach to post-market clinical follow-up studies.
Conclusion
In conclusion, post-market clinical follow-up (PMCF) studies are integral for confirming the safety and efficacy of medical devices in the market. These studies bridge the gap between pre-market testing and long-term performance, capturing valuable data on diverse patient populations.
Through PMCF studies, comprehensive data is collected, including details about device type, manufacturer, brand, and lot number, as well as any device-related problems. Vigilant reporting of device malfunctions, defects, and adverse events is crucial for maintaining safety.
In Latin America, regulatory oversight and post-market surveillance are paramount for ensuring the safety and effectiveness of medical devices. Compliance with rigorous standards, including tracking and examining adverse events, demonstrates a commitment to patient welfare and regulatory authorities.
The design of PMCF studies requires careful planning, considering factors such as study population, inclusion and exclusion criteria, duration of follow-up, and robust data collection methods. Ethical, legal, and social considerations must be prioritized, understanding market incentives and intellectual property rights.
In the growing Latin American medical device market, the execution of PMCF studies is essential. These studies contribute to improved healthcare outcomes and align with broader social goals. The industry's focus on precise remote data acquisition, as seen in discussions on Digital Health Technologies, highlights the importance of meticulous approaches to post-market clinical follow-up. Overall, PMCF studies play a critical role in ensuring the ongoing safety and efficacy of medical devices. By addressing the evolving regulatory landscape and benefiting patients and healthcare systems, these studies contribute to the advancement of medical device safety in Latin America.




