Introduction
Selecting the right site for a clinical trial is a complex process that involves considering various factors beyond just geographical location. Patients facing the opportunity to participate in groundbreaking research abroad, such as a patient from rural Pennsylvania with an ultra-rare disease considering a clinical trial in Turkey, encounter numerous logistical challenges like obtaining visas and navigating international travel.
Thoroughly evaluating patient accessibility and support infrastructure is crucial for successful enrollment and retention. This article explores the importance of site selection in clinical trials, taking into account practical considerations, patient eligibility, study design, ethical considerations, study timelines, partnerships, facilities and resources, and best practices. By prioritizing patient needs and regulatory requirements, researchers can enhance the likelihood of successful trial outcomes and advance medical knowledge.
Understanding the Importance of Site Selection
The intricacies of site selection for clinical trials extend far beyond mere geographical considerations. Take, for instance, a patient in rural Pennsylvania who is battling an ultra-rare disease without any FDA-approved treatments.
Presented with the chance to join a clinical trial in Turkey, the patient faces not just the prospect of participating in groundbreaking research but also the daunting challenge of navigating international travel. The complexity of obtaining visas, handling documentation in a foreign language, and arranging both air and ground transportation are just a few of the logistical hurdles that can arise. This scenario underscores the importance of thoroughly evaluating patient accessibility and support infrastructure when selecting trial sites to ensure successful enrollment and retention.
Practical and Protocol Considerations
When assessing potential sites for clinical trials, the implications of geographic location on patient participation are profound. For instance, a patient from rural Pennsylvania with a rare disease may face an arduous journey to partake in a trial situated in Turkey, grappling with challenges such as visa procurement, language barriers, and complex travel arrangements. Such scenarios underscore the importance of local participation, as mandated by components of the US Food and Drug Administration's Form 1572 (21 CFR 312.53[c]).
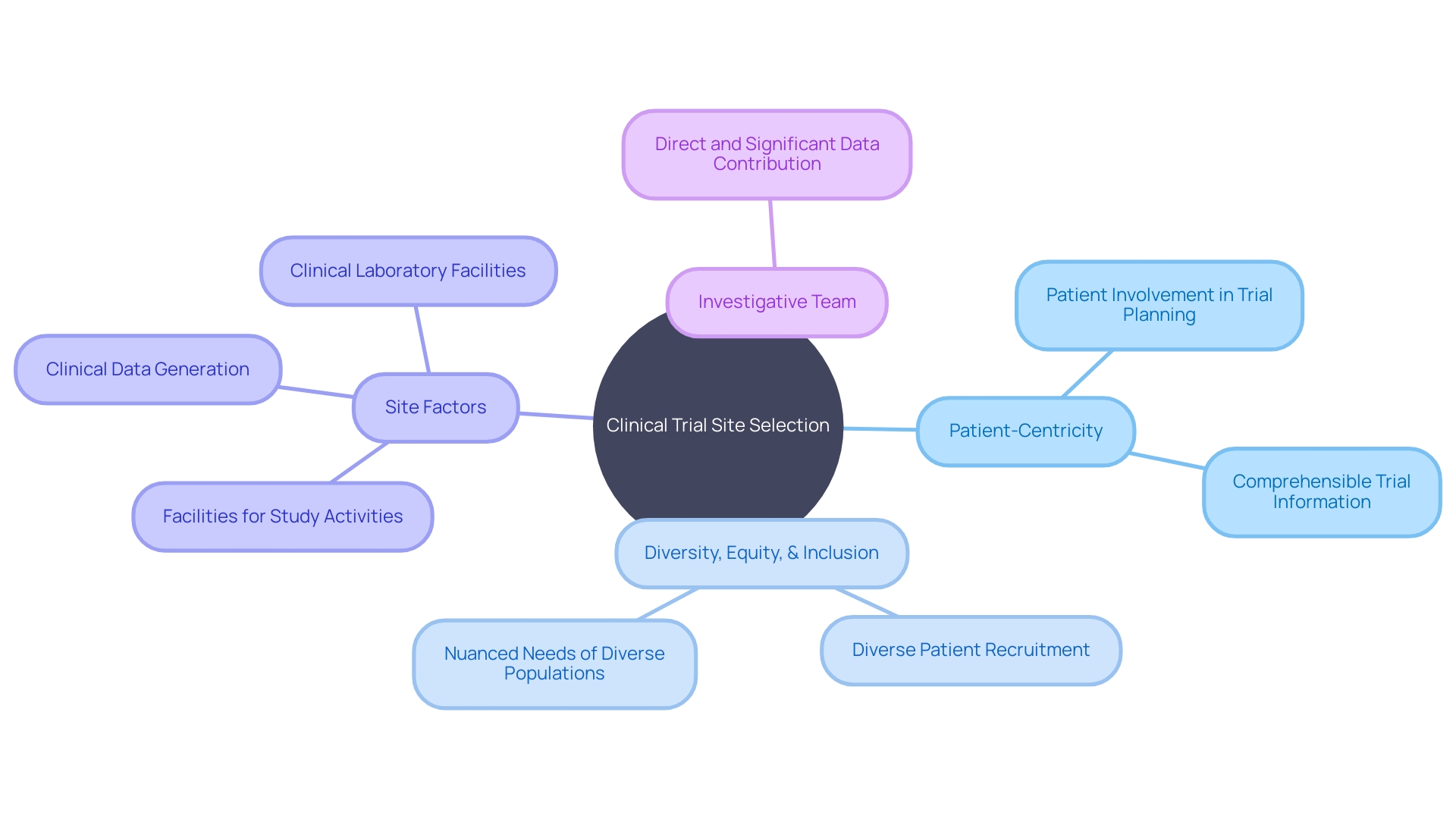
This regulation seeks to ensure that clinical trials are conducted at facilities capable of meticulous study execution and data collection, including medical schools, hospitals, and clinical laboratories. These facilities must not only be accessible but also equipped to uphold the study protocols, thereby facilitating the involvement of patients who can significantly advance the research outcomes. Hence, site selection transcends mere accessibility; it becomes a strategic decision that impacts patient inclusion, data integrity, and the overall feasibility of a clinical trial.
Population Profiles and Patient Eligibility
Selecting the right site for a clinical trial is a complex endeavor that involves delving into the unique characteristics of the patient population. For instance, consider the challenges faced by a patient in rural Pennsylvania who has a rare disease and is considering participating in a clinical trial abroad.
They must navigate the intricacies of international travel, such as obtaining visas and dealing with language barriers, which underscores the importance of patient-centric trial design. Daniel J Herron, an expert in digital offerings for regulated industries, emphasizes that patient-centricity means 'actively involving patients in the planning and design of trials, so their perspectives and needs are considered.'
It's about ensuring that trial information is comprehensible and accessible to participants. Moreover, incorporating diversity, equity, and inclusion principles is critical to reflect the varied ways individuals experience disease, shaped by factors including race, ethnicity, age, gender, and sexual orientation. These considerations are vital in identifying clinical trial sites that can not only recruit eligible participants effectively but also address the nuanced needs of a diverse patient population.
Study Design Assessment and Complexity
Selecting the right site for a clinical trial is a nuanced process that must take into account the intricacies of the study's design. The level of complexity can vary substantially, from straightforward observational studies to multifaceted interventional trials.
To ensure that a trial progresses without complications, it is imperative to align the site's infrastructure and the research team's expertise with the demands of the study. Consider, for instance, a patient from a rural area in Pennsylvania suffering from a rare disease with no approved treatments.
They are presented with a chance to join a clinical trial in Turkey, which could be potentially life-saving. Despite their willingness to participate, the patient faces logistical hurdles of international travel such as visa procurement, navigating foreign bureaucracy, and language barriers.
These challenges underscore the importance of local participation in clinical trials. The criteria set forth by the US Food and Drug Administration Form 1572 highlight the necessity of aligning the study's activities with the capabilities of the facilities where the trials are conducted. This includes specifying the medical institutions and clinical laboratories involved in the study, ensuring the generation and collection of clinical data are handled efficiently, and confirming that the investigative team can significantly contribute to the study's data. Matching the right side to the trial's requirements is not only crucial for adherence to the protocol but also for facilitating patient participation and the overall success of the clinical research.
Ethical Considerations and Vulnerable Populations
In the realm of clinical trials, the selection of appropriate sites is paramount, particularly when it involves vulnerable populations. These sites are not only required to uphold the highest ethical standards to safeguard participant rights and welfare but also need the expertise to handle the unique needs of groups such as children, pregnant women, and individuals with cognitive impairments.
A poignant example of the need for ethical consideration is the scenario of a rural Pennsylvania patient with an ultra-rare disease who is offered participation in a clinical trial in Turkey. The logistical challenges of cross-border travel raise numerous ethical questions, including visa acquisition, language barriers for document handling, and coordination of travel arrangements.
Moreover, the profound impact of advocates like Terry Moakley, who championed for disability access, highlights the imperative for clinical trial sites to be inclusive and cater to participants with disabilities. The ethical landscape of clinical trials is further complicated by the broader environmental and societal impacts.
The World Health Organization has cited 'air pollution and climate change' as the top global health threat since 2019, and clinical trials are significant contributors to greenhouse gas emissions. With an estimated 27.5 million tons of carbon emissions from clinical trials registered on ClinicalTrials. Gov alone, the ethical considerations extend beyond immediate participant welfare to the long-term effects on global health, including the potential for 250,000 additional deaths per year between 2030 and 2050 from climate-related issues. These considerations are crucial for the scientific community, policymakers, IRBs, and ethics committees to take into account when evaluating the potential benefits and risks of clinical research.
Study Timelines and Objectives
Selecting the right site for a clinical study is a critical step that goes beyond basic logistics; it must be a strategic decision that reflects the study's unique timelines, objectives, and participant needs. This consideration is particularly acute in scenarios where patients face significant barriers to participation, such as geographical limitations and logistical challenges. Take, for instance, a patient in rural Pennsylvania with an ultra-rare disease.
The chance to join a clinical trial in Turkey could be a lifeline, yet the complexities of international travel, from visa procurement to navigating paperwork in a foreign language, pose daunting hurdles. It's imperative that site selection not only looks at the capacity for enrolling the necessary participants within the given timeline but also takes into account the ease of access for participants, ensuring that the objectives of the site and the study are in sync. A collaborative approach between the site and study sponsors can help in crafting a participant-centric strategy that addresses these challenges, ultimately contributing to the success and integrity of the clinical trial.
Partnerships, Competitors, and Staffing
In the meticulous process of site selection for clinical trials, one must adhere to the stringent components of the US Food and Drug Administration Form 1572, which emphasizes the importance of enabling patient participation at a local level. This involves the careful identification of medical schools, hospitals, and other research facilities where significant study activities and data collection will occur, as well as clinical laboratory facilities that will contribute to the study.
Starting early in this process is vital, especially considering the vast number of rare diseases—each with unique characteristics and specific needs in terms of measurement—that require attention. Despite improvements in funding for natural history work, the challenge remains to implement broad approaches that accelerate learning across many diseases simultaneously.
Emphasizing patient-centricity, as highlighted by Daniel J Herron of RWS, involves prioritizing the patient experience by involving them in trial planning and ensuring they receive comprehensible information. This approach necessitates a focus on diversity, equity, and inclusion to capture the varying lived experiences and conditions of individuals across different demographics. Such comprehensive and patient-focused strategies in site selection not only streamline the process but also strengthen the research network and ensure the site's capability to meet the study's demands.
Facilities and Resources
The selection of clinical trial sites is a critical step that demands careful consideration of several factors to ensure the trial's success. It is imperative that the chosen site possesses the necessary infrastructure, including clinical facilities, laboratories, and secure storage areas for trial materials. Additionally, a robust staff comprising research coordinators, nurses, and physicians is essential for the seamless execution of the trial.
A poignant example of the significance of local site selection is the case of a patient from rural Pennsylvania with a rare disease and no approved treatments. Offered a place in a pivotal trial in Turkey, the patient faces daunting challenges such as securing visas, navigating foreign paperwork, and arranging travel. These logistical hurdles underscore the importance of the US FDA Form 1572's requirement to detail the locations where clinical activities and data collection will occur.
This regulation ensures that clinical laboratories and research facilities contributing to the study are clearly identified, facilitating patient participation closer to home. As articulated by industry experts, many companies recognize in hindsight that earlier phases of their studies could have been designed more effectively. A thorough, proactive approach in planning and site selection can prevent such oversights, ultimately enhancing the trial's efficiency and outcomes.
Best Practices for Clinical Site Selection
The process of clinical trial site selection is a multifaceted endeavor that extends beyond the mere identification of locations. It encompasses a deep consideration of patient-centric factors, such as the case of a patient in rural Pennsylvania with an ultra-rare disease.
When the only available clinical trial is in Turkey, the patient faces a daunting array of logistical challenges, from obtaining visas to navigating foreign paperwork, all of which underscore the need for more accessible trial sites. Clinical trial companies must diligently assess the feasibility and ethical implications of site selection, ensuring that participants can reasonably access trials without undue burden.
This is highlighted by the complexities outlined in the US FDA Form 1572, which mandates the clear identification of facilities for study activities and data collection, as well as the involvement of laboratories and team members who significantly contribute to the research. By prioritizing the selection of trial sites that are considerate of patient needs and regulatory requirements, researchers can bolster the likelihood of successful trial outcomes. This strategic approach not only serves the interests of patients but also aligns with the overarching objective of clinical trials: to advance medical knowledge and treatment options for those in need. The integration of patient accessibility into site selection criteria is not just a best practice; it is a compassionate and essential component of patient-centered research.
Conclusion
In conclusion, selecting the right site for a clinical trial involves thorough evaluation of practical considerations, patient eligibility, study design, ethical considerations, study timelines, partnerships, facilities and resources, and best practices. Patient accessibility and support infrastructure play a crucial role in successful enrollment and retention.
Prioritizing patient needs and regulatory requirements enhances the likelihood of successful trial outcomes. This approach advances medical knowledge and treatment options while ensuring compassionate and essential patient-centered research practices.




