Introduction
Navigating the regulatory landscape for medical devices in Peru involves understanding the intricate framework established by key health institutions. The National Institute of Health (INS) and the Ministry of Health (MINSA) play pivotal roles in ensuring that medical devices meet stringent safety, efficacy, and quality standards before entering the market. This alignment with global regulatory practices is crucial for maintaining high-quality healthcare systems and protecting public health.
Additionally, the regulatory environment in Peru is structured to support rigorous clinical research through stringent guidelines and ethical oversight, which are essential for the safety and performance of medical devices. This article delves into the regulatory framework, the role of key regulatory bodies, the requirements for conducting clinical trials, and the ethics committee approval process, providing a comprehensive overview of how medical devices are governed in Peru.
Regulatory Framework for Medical Devices in Peru
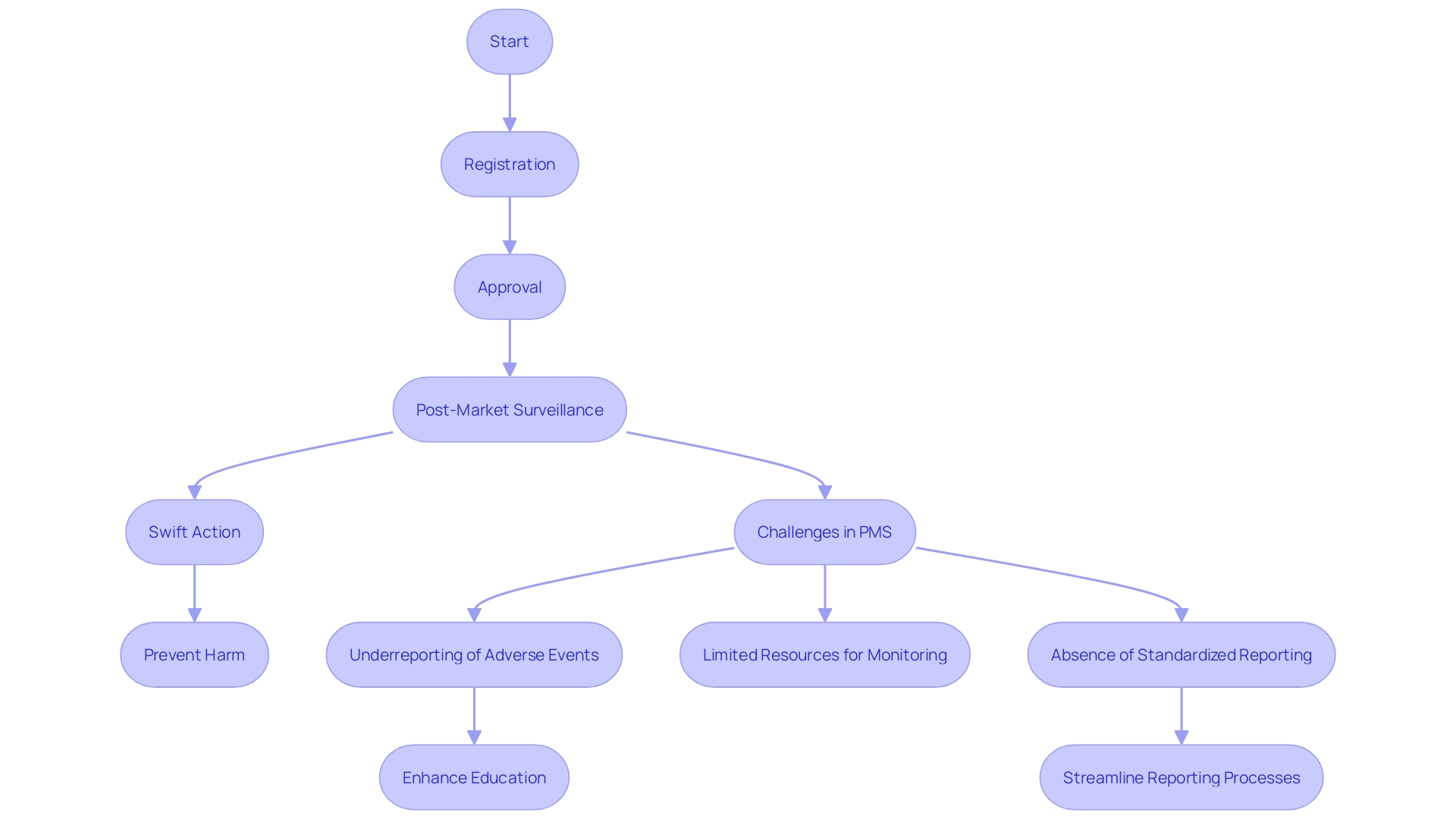
The regulatory structure for medical equipment in Peru is mainly supervised by the National Institute of Health (INS) and the Ministry of Health (MINSA). These institutions create comprehensive legal and procedural guidelines for the registration, approval, and post-market surveillance of medical instruments. This framework ensures that medical instruments meet stringent safety, efficacy, and quality standards before they are introduced to the market, aligning with global regulatory practices. The harmonization with international standards facilitates smoother market integration and compliance, reflecting similar approaches seen with agencies like the FDA in the United States and EMA in Europe. This alignment is crucial as it supports the maintenance of high-quality healthcare systems needed for the overall wellbeing of communities, which is particularly vital given that poor-quality healthcare contributes to 60% of deaths from treatable conditions in low- and middle-income countries annually. Furthermore, the regulatory framework in Peru includes strict control mechanisms to ensure that medical products, including implantable ones like cardiac pacemakers and artificial joints, are thoroughly evaluated for their intended use and technological complexity, thereby safeguarding public health.
Key Regulatory Bodies Involved in Clinical Research for Medical Devices
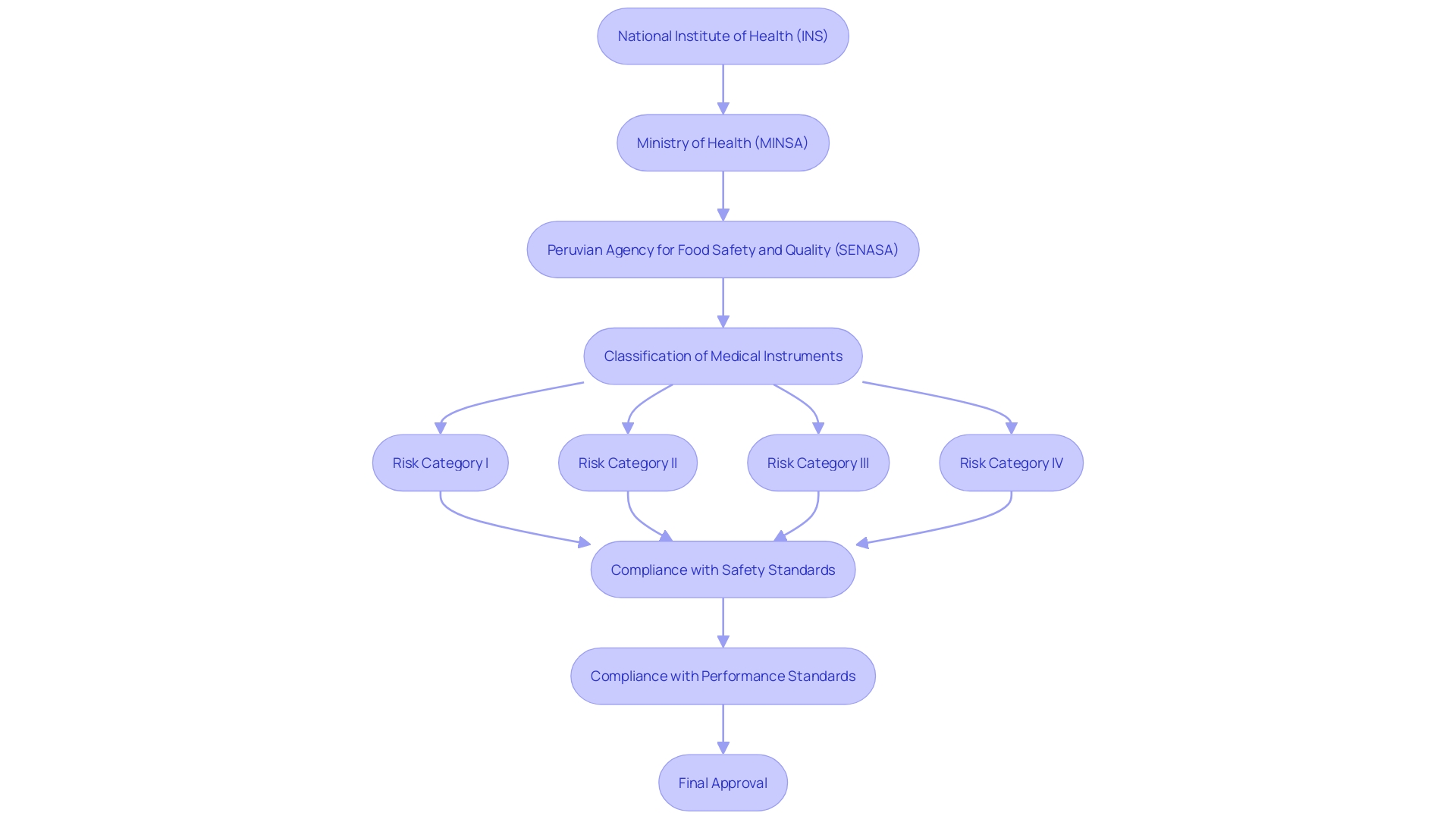
In Peru, the regulatory framework for medical instruments is overseen by several key organizations, each playing an essential role in ensuring the safety and effectiveness of these instruments. The National Institute of Health (INS) is mainly accountable for the assessment and authorization of clinical trials related to medical equipment. The INS functions within a framework that classifies items into four risk categories, ensuring suitable regulatory measures are established according to the risk level.
The Ministry of Health (MINSA) provides additional regulatory oversight, ensuring compliance with national health policies. This dual-layered approach helps maintain rigorous standards for safety and performance of the equipment. Moreover, the Peruvian Agency for Food Safety and Quality (SENASA) may also take part in the regulatory process, especially when the intended application relates to food safety and quality issues.
Peru's regulatory environment is aligned with global standards, which are essential for guaranteeing the quality, safety, efficacy, and performance of medical products before they reach the market. This alignment helps safeguard public health by preventing, enhancing, and maintaining high standards. As noted in international studies, ensuring compliance with these regulations is becoming increasingly challenging due to evolving legislation and the growing volume of literature to review. Nonetheless, these strict actions are essential for the ongoing confidence and dependability of medical instruments utilized in healthcare environments.
Requirements for Conducting Clinical Trials in Peru
Carrying out research studies for medical devices in Peru necessitates following particular and strict regulations. Researchers must submit a thorough trial application that includes study protocols, informed consent forms, and data management plans, ensuring all moral and regulatory standards are met. For instance, the ethics committees of the regional health authority of Cajamarca and the UPCH approved a recent study, emphasizing the necessity of thorough oversight (Ref. 268-12-15).
Applications are meticulously reviewed by ethics committees and regulatory bodies to ensure compliance and ethical integrity. This is crucial as discrepancies between intended medical objectives and actual outcomes can arise if endpoints are not well-defined, underscoring the importance of precise data collection.
Furthermore, investigators must strictly adhere to Good Clinical Practice (GCP) guidelines throughout the study to ensure participant safety and data integrity. 'This adherence is paramount, as aligning a product's market expectations with its therapeutic benefits requires factual evidence and robust data, as highlighted by regulatory bodies like the EU MDR and MDCG.'. These regulations require that assertions regarding a product's safety and effectiveness are backed by solid data, ensuring clarity and precision in research studies.
Ethics Committee Approval Process
The ethics committee authorization procedure in Peru is a cornerstone for starting medical research involving medical devices. Each clinical study must obtain clearance from an Institutional Review Board (IRB) or ethics committee, which involves a thorough review of the study’s objectives, methodology, and potential risks to participants. This approval process ensures that the trial meets rigorous moral standards, safeguarding the rights and welfare of participants as well as ensuring scientific validity.
IRBs play a vital role in protecting participants by following fundamental research ethics principles such as respect for persons, beneficence, and justice. However, the review process can often become more about compliance than the fundamental commitments, leading to a conservative approach in approving protocols. This tendency is compounded by the fact that IRBs are frequently inundated with protocols and under-resourced, relying on heuristics to guide their decisions.
The significance of moral supervision is further emphasized by global instances. For instance, in Vietnam, the Ministry of Health oversees clinical trials, but cultural factors like language barriers and literacy rates can influence participant perceptions of research ethics. In contrast, in Laos, research is often financed by external contributors with little national supervision, raising moral concerns. These examples demonstrate the complexities and challenges in upholding moral standards across various regions.
'Furthermore, recent progress in review procedures, such as the UK's Medicines and Healthcare products Regulatory Agency (MHRA) new system to speed up approvals for low-risk studies, illustrate the continuous endeavors to balance efficiency with moral integrity.'. Such measures aim to streamline the approval process, reducing the time for initial applications from 30 to 14 days for Phase III and IV low-risk studies.
In conclusion, the ethics committee approval process in Peru and other regions emphasizes the critical need for strong oversight in clinical trials. By adhering to established ethical standards and continuously improving the review processes, we can ensure the protection of participants and the integrity of scientific research.
Conclusion
The regulatory framework for medical devices in Peru is primarily governed by the National Institute of Health (INS) and the Ministry of Health (MINSA). These institutions enforce stringent safety, efficacy, and quality standards that align with international practices, thereby enhancing public health protection and facilitating market integration.
Key regulatory bodies oversee the evaluation and classification of medical devices based on their risk levels, ensuring that safety and performance standards are rigorously upheld. The Peruvian Agency for Food Safety and Quality (SENASA) also plays a role in addressing health policies related to medical devices.
For clinical trials, adherence to ethical and regulatory guidelines is essential. Ethics committees conduct thorough reviews to ensure participant welfare and the scientific validity of trials, with Good Clinical Practice (GCP) guidelines ensuring data integrity and safety.
The ethics committee approval process is crucial for maintaining ethical standards in clinical research. While challenges exist, such as resource limitations, ongoing improvements aim to streamline the approval process while safeguarding participant rights. Overall, Peru's regulatory landscape is vital for ensuring the safety and efficacy of medical devices, ultimately contributing to the health and wellbeing of its population.




