Overview
The article highlights the critical strategies required to enhance patient diversity in Bolivian clinical trials, underscoring its significance for generating relevant and trustworthy medical outcomes. It elaborates on the necessity for:
- Targeted recruitment
- Strict adherence to regulatory guidelines
- Ongoing monitoring of diversity initiatives
These elements collectively ensure that clinical research accurately reflects and addresses the needs of the entire population, thereby reinforcing the credibility and applicability of medical findings.
Introduction
In the realm of clinical research, the importance of patient diversity cannot be overstated. As the medical community increasingly recognizes that treatments can affect individuals differently based on their genetic, environmental, and lifestyle backgrounds, the push for inclusive clinical trials has gained momentum.
Despite this awareness, a significant gap remains in the representation of diverse populations, which can lead to incomplete data and inequitable healthcare outcomes. From targeted recruitment strategies to compliance with regulatory guidelines, the journey toward achieving diversity in clinical trials is multifaceted and essential for building trust within underrepresented communities.
As the landscape of medical research evolves, it becomes imperative to not only acknowledge the challenges but also to implement effective measures that foster inclusivity and ensure that all voices are heard in the quest for better health solutions.
Recognize the Importance of Patient Diversity in Clinical Trials
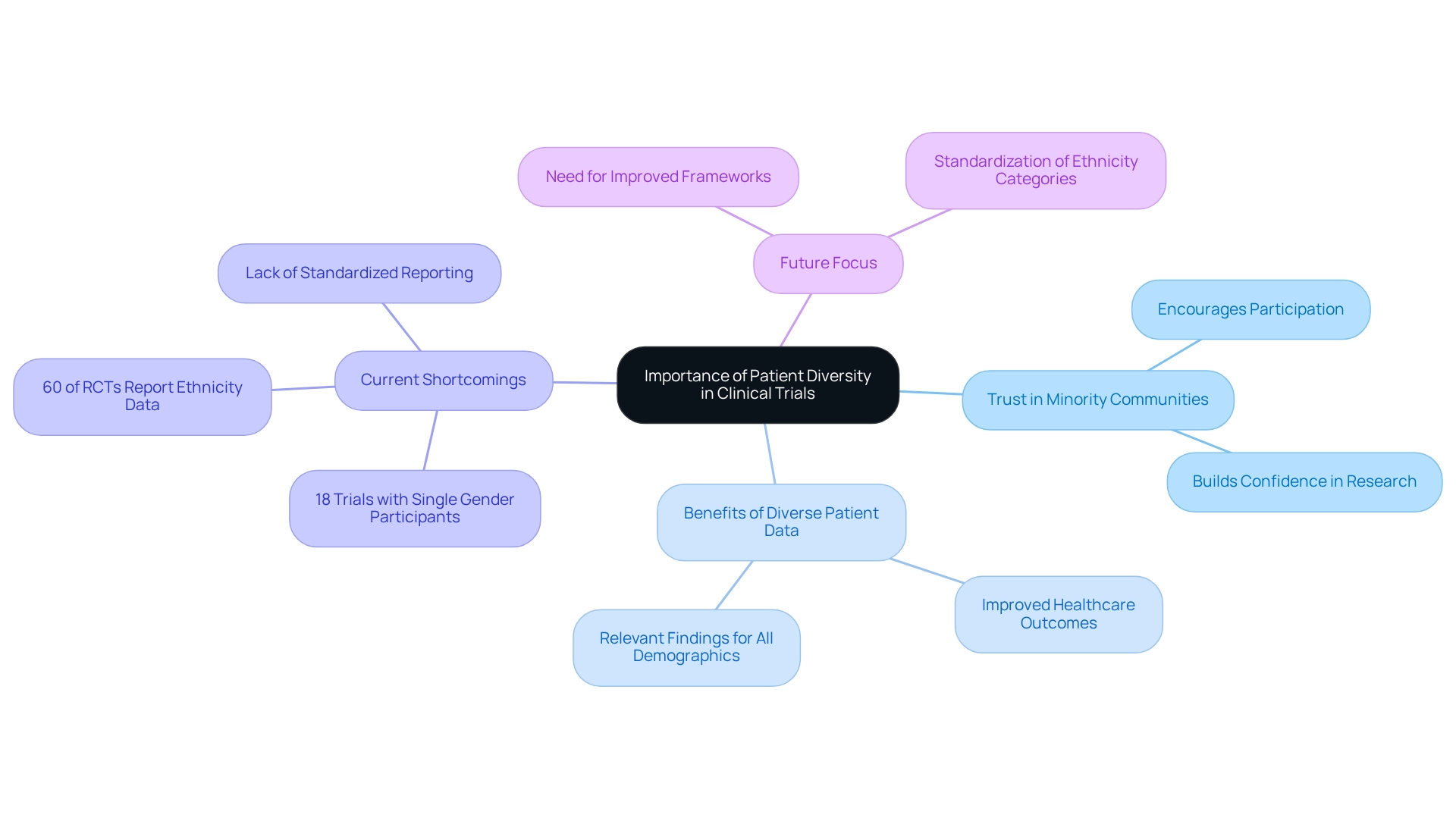
Having patient diversity in Bolivian clinical trials is essential for producing trustworthy and relevant outcomes across different demographic groups. Due to genetic, environmental, and lifestyle factors, patient diversity in Bolivian clinical trials can result in different responses to treatments. A notable research study emphasized that patient diversity in Bolivian clinical trials is essential, as medical studies lacking this variety may overlook crucial safety and effectiveness information pertinent to underrepresented populations. For instance, there were 18 experiments that enrolled individuals of one gender, highlighting the existing limitations in variety within medical studies. By emphasizing inclusion and patient diversity in Bolivian clinical trials, researchers can ensure their findings are relevant to the entire population, ultimately leading to enhanced healthcare outcomes.
Moreover, patient diversity in Bolivian clinical trials encourages confidence in health research within minority communities, which have traditionally been underrepresented. This trust is vital for promoting involvement and ensuring that patient diversity in Bolivian clinical trials accurately reflects the demographics of the populations they seek to assist. Recent analyses indicate that only 60% of randomized controlled trials (RCTs) reported ethnicity data, underscoring the variability in how ethnicity is documented across studies. As Arthur L. Caplan, Professor of Bioethics, observed, "pointing toward the availability of studies as an advantage to justify efforts at inclusion when so many Americans are uninsured or burdened with overwhelming medical debt that prevents them from accessing the advancements of studies makes even less sense." Standardizing these categories is essential for significant comparisons with national demographic data and improving the inclusivity of medical studies. Furthermore, the absence of guidance from NIHR on collecting data related to inclusion underscores the obstacles encountered in attaining representation in medical studies, stressing the necessity for improved frameworks. As we approach 2025, the focus on patient diversity in Bolivian clinical trials will be more crucial than ever, not only for ethical reasons but also for the integrity and relevance of research results.
Implement Targeted Recruitment Strategies for Diverse Populations
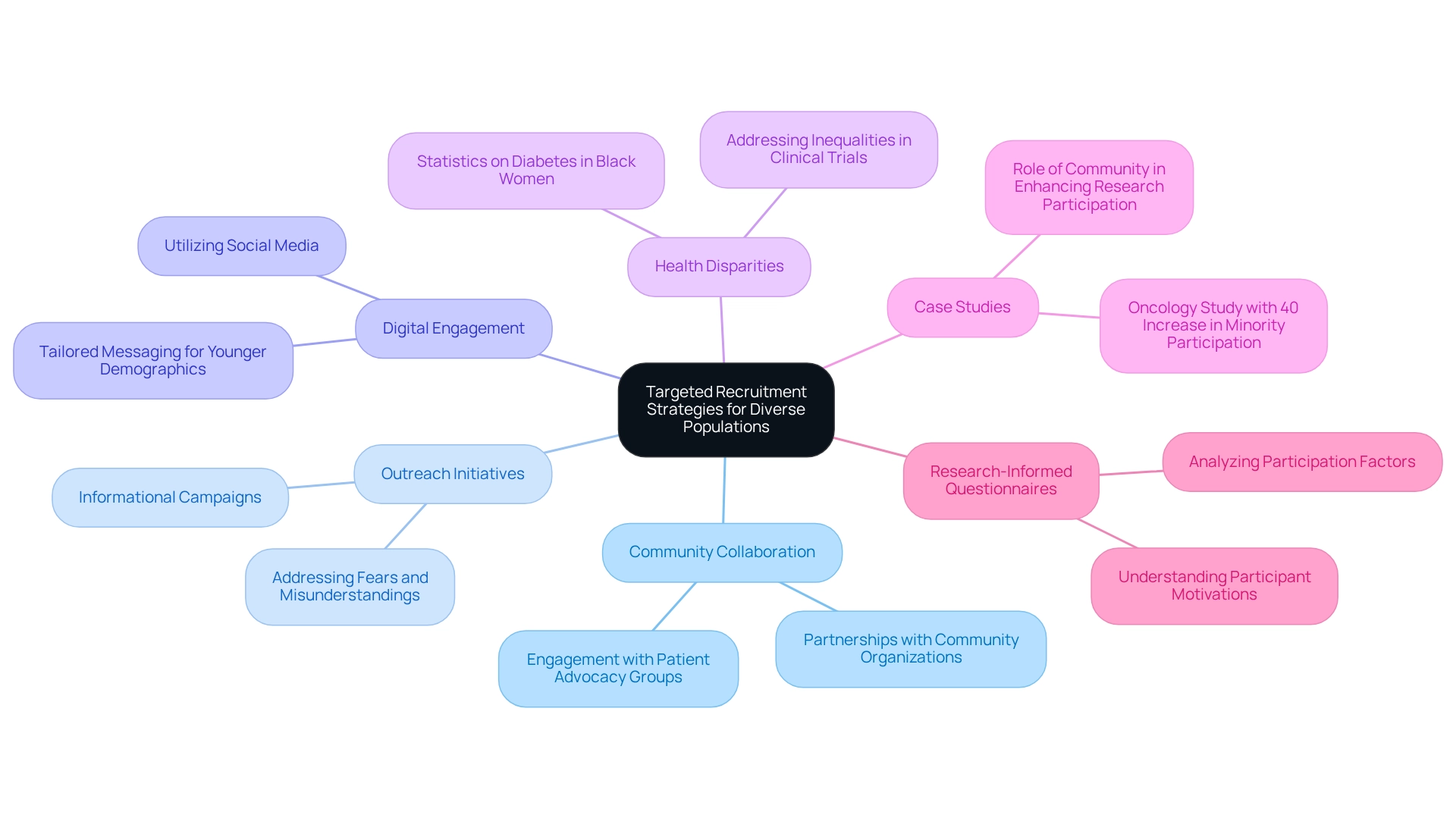
To effectively enlist varied groups for research studies, it is essential to apply focused recruitment methods that acknowledge patient diversity in Bolivian clinical trials. Collaborating with community organizations, healthcare providers, and patient advocacy groups that have established trust within specific demographics significantly enhances recruitment efforts, particularly regarding patient diversity in Bolivian clinical trials. Outreach initiatives designed to inform prospective participants about the advantages and safety of research studies assist in alleviating fears and misunderstandings that might obstruct participation.
Moreover, leveraging digital platforms and social media expands outreach, particularly among younger demographics. Tailoring messaging to resonate with various cultural backgrounds and languages further enhances engagement. A notable case study from a recent oncology study revealed that targeted recruitment initiatives resulted in a remarkable 40% increase in minority participation, which highlights the importance of patient diversity in Bolivian clinical trials and underscores the effectiveness of these strategies.
Additionally, recognizing health disparities is crucial; for instance, Black women are 60 percent more likely to develop or be diagnosed with diabetes following breast cancer treatment than white women. This statistic emphasizes the necessity for patient diversity in Bolivian clinical trials to successfully tackle such inequalities.
A research-informed questionnaire has been developed to examine factors influencing participation in studies among patients, highlighting the significance of comprehending participant motivations and obstacles. Furthermore, a participant from the LGBTQ+ community noted, "I would identify my community as the LGBTQ+ community…and the chronic illness/disabled community," illustrating the necessity of recognizing diverse communities in recruitment efforts.
The case study titled 'Role of Community in Enhancing Research Participation' also highlights how utilizing community resources and support can greatly enhance recruitment and retention of diverse participants, thereby improving patient diversity in Bolivian clinical trials. By promoting community involvement and employing creative outreach strategies, studies can attain broader representation and inclusivity, ultimately resulting in more thorough and representative findings.
Leverage Regulatory Guidelines to Enhance Diversity Efforts
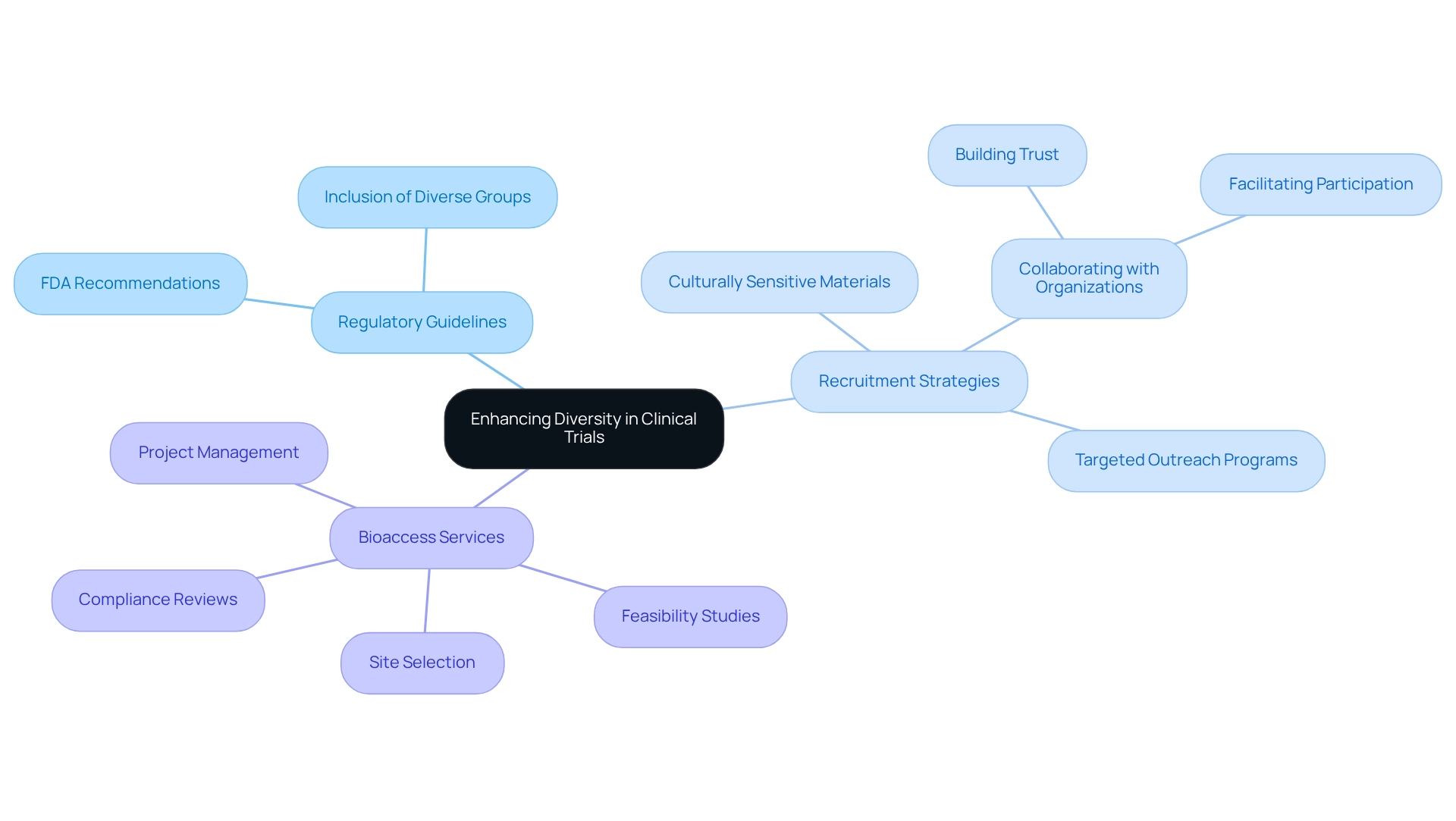
Improving variety in medical studies is fundamentally connected to compliance with regulatory standards, particularly those established by entities like the FDA. These guidelines advocate for the inclusion of diverse groups in health studies, highlighting the significance of patient diversity in Bolivian clinical trials while outlining optimal methods for participant recruitment and retention. The FDA's recent recommendations specifically urge sponsors to formulate strategies aimed at recruiting underrepresented groups to enhance patient diversity in Bolivian clinical trials, including developing targeted outreach programs to engage communities that are typically underrepresented in research studies.
- Collaborating with local organizations and healthcare providers to build trust and facilitate participation.
- Implementing culturally sensitive recruitment materials and processes to ensure inclusivity.
By aligning study designs with these regulatory expectations, researchers can greatly enhance patient diversity in Bolivian clinical trials, thereby improving the quality and applicability of their findings. This approach not only addresses the ethical imperative of equity in medical research but also aligns with broader social justice goals, emphasizing the importance of patient diversity in Bolivian clinical trials to ensure that medical devices and treatments benefit a wider population. Furthermore, adherence to these guidelines fosters greater trust among stakeholders, including patients and regulatory bodies.
For instance, bioaccess offers extensive research management services that encompass feasibility studies, site selection, compliance reviews, setup, import permits, project management, and reporting. Each of these services plays a crucial role in enhancing variety: feasibility studies help identify diverse populations, site selection ensures experiments are conducted in accessible locations, compliance reviews guarantee adherence to ethical standards, and project management facilitates effective communication with underrepresented communities. By improving access to information and resources, organizations can boost participation from diverse populations, which is essential for achieving patient diversity in Bolivian clinical trials. By applying these regulatory suggestions and leveraging bioaccess's expertise, organizations can create a more inclusive research environment that ultimately enhances patient diversity in Bolivian clinical trials, resulting in safer and more effective medical innovations for all.
Monitor and Evaluate Diversity Initiatives for Continuous Improvement
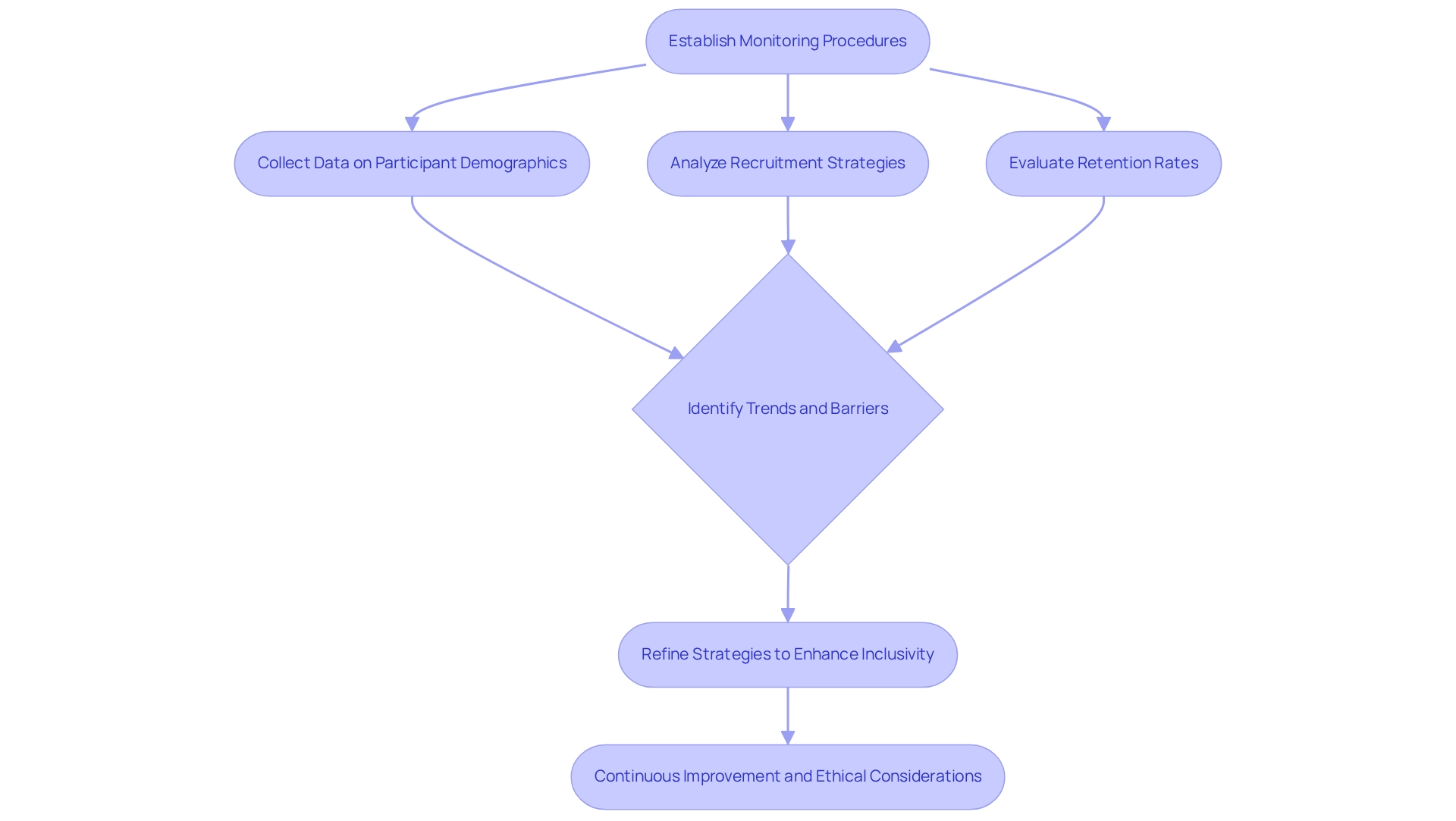
Establishing robust monitoring and assessment procedures is imperative for the success of inclusion initiatives in medical studies, especially in promoting patient diversity in Bolivian clinical trials. This involves the systematic collection and analysis of data related to participant demographics, recruitment strategies, retention rates, and patient diversity in Bolivian clinical trials. By scrutinizing these metrics, organizations can uncover trends and identify barriers that may impede patient diversity in Bolivian clinical trials efforts.
For instance, a medical study that initially focuses on patient diversity in Bolivian clinical trials may discover that certain demographic segments remain underrepresented despite focused outreach. By rigorously evaluating the recruitment process, researchers can refine their strategies—such as enhancing community engagement or modifying eligibility criteria to promote inclusivity.
Continuous improvement driven by assessment outcomes not only enhances the quality of medical studies but also significantly contributes to patient diversity in Bolivian clinical trials, playing a critical role in advancing equitable healthcare results. The FDA's observation that 80 percent of authorized applications for drugs and biologics included data from studies conducted outside the United States highlights the necessity of patient diversity in Bolivian clinical trials to generate comprehensive and relevant findings.
Moreover, case studies, such as the PRGLAC report's recommendations for engaging pregnant and lactating individuals in research trials, highlight the necessity of specialized informed consent and medical clearance. Implementing these suggestions can yield better health outcomes and more informed prescribing practices, further illustrating the significance of patient diversity in Bolivian clinical trials within medical research.
As a study coordinator remarked, "So I think it is the relationship and trust to me is the key. Once trust is established, people will do things that I believe are coming from you and you better keep that promise." This statement underscores the importance of cultivating trust within diverse communities to bolster recruitment efforts, particularly for patient diversity in Bolivian clinical trials.
Additionally, it is essential to acknowledge ongoing efforts to address historical and contemporary injustices in medical studies. By integrating these considerations into assessment procedures, organizations can ensure that their inclusion initiatives address patient diversity in Bolivian clinical trials, making them not only effective but also ethically sound.
To implement these initiatives successfully, organizations should contemplate specific evaluation metrics for inclusiveness in medical studies, such as:
- Patient diversity in Bolivian clinical trials
- Participant demographic representation
- Recruitment success rates
- Retention statistics
These metrics will provide actionable insights for clinical research directors seeking to enhance diversity in their trials.
Conclusion
Diversity in clinical trials is not merely a goal; it is a cornerstone of ethical and effective medical research. The article underscores that varying responses to treatments among different demographic groups can result in incomplete data and inequitable healthcare outcomes. By fostering trust among underrepresented populations, we enhance participation, ensuring that research findings remain relevant and beneficial to all.
Implementing targeted recruitment strategies stands as a critical approach to bridging the diversity gap. Collaborating with community organizations and employing culturally sensitive outreach methods can significantly elevate representation, as demonstrated by successful initiatives that have increased minority participation. This approach enriches the research process by integrating diverse experiences and perspectives.
Furthermore, adherence to regulatory guidelines plays an essential role in amplifying diversity efforts. By aligning research practices with established recommendations, organizations can cultivate inclusive environments that facilitate the recruitment and retention of underrepresented groups. Such a commitment not only fulfills ethical obligations but also builds trust with stakeholders, paving the way for equitable healthcare solutions.
Continuous monitoring and evaluation of diversity initiatives are imperative for sustained improvement. By analyzing participant demographics and recruitment strategies, organizations can identify barriers and refine their approaches, thereby ensuring that diversity initiatives are both effective and ethically sound.
In conclusion, achieving diversity in clinical trials necessitates collaborative efforts among researchers, regulatory bodies, and community organizations. Prioritizing this imperative will yield more comprehensive research outcomes and contribute to a healthier, more equitable society for everyone.
Frequently Asked Questions
Why is patient diversity important in Bolivian clinical trials?
Patient diversity is essential in Bolivian clinical trials because it leads to trustworthy and relevant outcomes across different demographic groups, ensuring that treatments are effective and safe for all populations.
What are the consequences of lacking patient diversity in medical studies?
Without patient diversity, medical studies may overlook critical safety and effectiveness information pertinent to underrepresented populations, which can result in biased or incomplete research findings.
What did a notable research study reveal about gender representation in clinical trials?
The study highlighted that 18 experiments enrolled individuals of only one gender, indicating significant limitations in diversity within medical studies.
How does patient diversity affect trust in health research within minority communities?
Encouraging patient diversity helps build confidence in health research among minority communities, which have traditionally been underrepresented, promoting their involvement in clinical trials.
What percentage of randomized controlled trials (RCTs) reported ethnicity data?
Recent analyses indicated that only 60% of randomized controlled trials reported ethnicity data, highlighting variability in how ethnicity is documented across studies.
What is the significance of standardizing categories for ethnicity in clinical trials?
Standardizing categories is crucial for making significant comparisons with national demographic data and improving the inclusivity of medical studies.
What challenges exist in achieving representation in medical studies?
The absence of guidance from organizations like NIHR on collecting data related to inclusion highlights the obstacles faced in attaining representation in medical studies.
Why will patient diversity in Bolivian clinical trials be increasingly important as we approach 2025?
As we approach 2025, the focus on patient diversity will be crucial for ethical reasons and for ensuring the integrity and relevance of research results.




