Overview
Patient diversity in Paraguay's clinical trials is essential for ensuring that research findings accurately reflect the entire population, guaranteeing that medical devices are both safe and effective across various demographics. This article emphasizes the significance of inclusive study designs, community engagement, and tailored recruitment strategies. These elements collectively enhance participation and foster trust among diverse groups, ultimately elevating the quality and relevance of clinical research outcomes.
Introduction
In the realm of clinical research, the importance of patient diversity cannot be overstated. As the medical landscape evolves, it is essential to ensure that clinical trials reflect the varied demographics of the population. This reflection is crucial for developing treatments that are safe and effective for all. Diverse representation in trials not only enhances the validity of research outcomes but also addresses historical disparities in healthcare.
With regulatory bodies emphasizing the need for inclusivity, the challenge lies in understanding the barriers to participation and implementing effective strategies to engage underrepresented communities. By fostering trust and collaboration, researchers can unlock the potential of diverse patient populations, paving the way for groundbreaking advancements in medical science.
Understand the Importance of Patient Diversity in Clinical Trials
Understanding patient diversity in Paraguay clinical trials is essential for ensuring that the findings reflect the entire population rather than just specific demographics. Patient diversity in Paraguay clinical trials is essential as it allows researchers to gain insights into how different groups respond to treatments, which is crucial for the development of safe and effective medical devices. For example, genetic, social, and cultural factors can significantly influence drug metabolism and effectiveness, underscoring the importance of including a wide range of participants to minimize biases and enhance the quality of medical research.
Recent studies, such as the PLATINUM Diversity study, whose primary results were published in October 2017 in JAMA Cardiology, have reinforced the necessity of this diversity, revealing that approximately 1 in 5 new molecular entities approved by the FDA exhibit varying impacts on different racial and ethnic groups. This statistic highlights the critical need for inclusive study designs that genuinely reflect patient diversity in Paraguay clinical trials.
Moreover, regulatory organizations are increasingly prioritizing patient diversity in Paraguay clinical trials, recognizing it as vital for effective study design and execution. As experts in the field have noted, "We will only attract a more varied group of patients in studies when we appropriately comprehend the elements influencing choices regarding participation in research." Understanding these factors is key to drawing a more diverse patient pool. Historical case studies, such as the Tuskegee Syphilis Experiment, illustrate the lingering skepticism towards medical institutions within communities of color, emphasizing the necessity for transparency and ethical practices in research involving patients.
To effectively promote patient diversity in clinical trials, consider the following steps:
- Engage with diverse communities to understand their concerns and motivations regarding participation in studies.
- Implement culturally sensitive recruitment strategies that resonate with various demographic groups.
- Ensure clarity about the process and the benefits of involvement to build trust.
- Offer education and resources to potential participants about the significance of diversity in health-related research.
In conclusion, patient diversity in Paraguay clinical trials not only enhances the reliability of research findings but also contributes to the development of medical devices that are effective across diverse populations. With bioaccess's expertise in managing comprehensive research services—including feasibility studies, site selection, compliance reviews, Early-Feasibility, and First-In-Human Studies—the emphasis on diverse patient representation can lead to improved health outcomes for all.
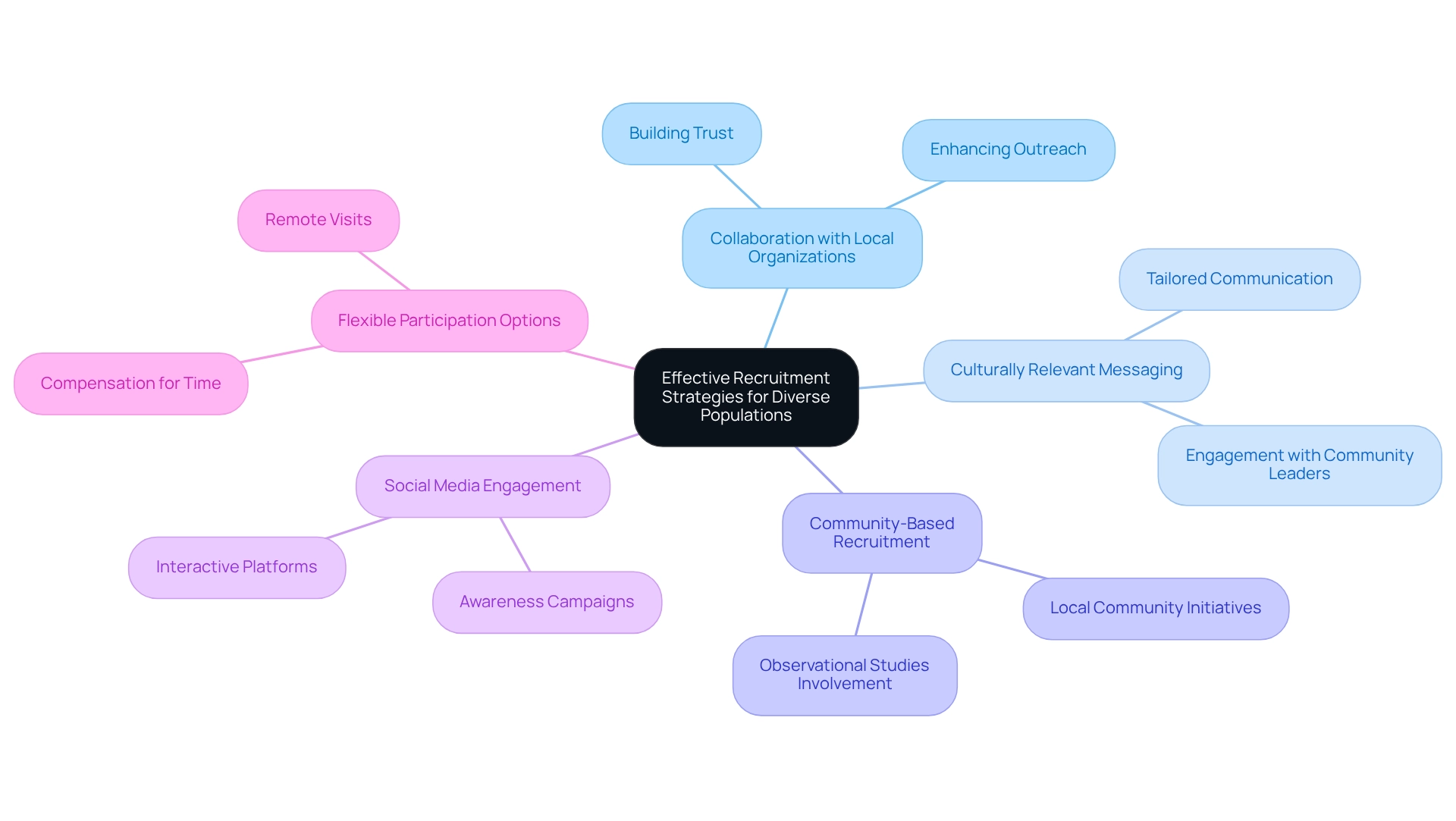
Implement Effective Recruitment Strategies for Diverse Populations
To successfully enlist varied groups for medical studies, sponsors must implement focused recruitment strategies that promote patient diversity in Paraguay clinical trials. Collaborating with local organizations that support underrepresented groups is crucial for achieving patient diversity in Paraguay clinical trials, as these alliances build trust and enhance outreach. By utilizing culturally relevant messaging and employing diverse recruitment staff who can connect with potential participants, we can enhance patient diversity in Paraguay clinical trials.
Additionally, leveraging social media channels and participating in local activities significantly raises awareness regarding patient diversity in Paraguay clinical trials among various groups. Offering flexible participation options, such as remote visits or compensation for time, helps mitigate barriers to involvement.
For instance, studies have shown that initiatives involving local community leaders experienced a notable rise in minority enrollment, which highlights the importance of community-based recruitment strategies for improving patient diversity in Paraguay clinical trials. This approach not only boosts participation rates but also contributes to the overall integrity and relevance of research outcomes.
With the worldwide research participant recruitment market projected to reach $5.45 billion by 2030, the importance of efficient recruitment strategies cannot be overstated. Camille Pope, Chief Medical Lead at Acclinate, emphasizes that "updated guidance recommends that companies submit their plans to the FDA for review before initiating a significant phase-three or pivotal study, which is important because it takes time to build effective trust and truly engage in a genuine manner with individuals you haven’t interacted with previously."
Understanding the historical context of skepticism in medical studies, especially regarding patient diversity in Paraguay clinical trials and the legacy of unethical treatment of marginalized groups, is vital for formulating strategies to restore confidence and enhance involvement in health studies. Involving various communities in observational studies may also boost overall research participation.
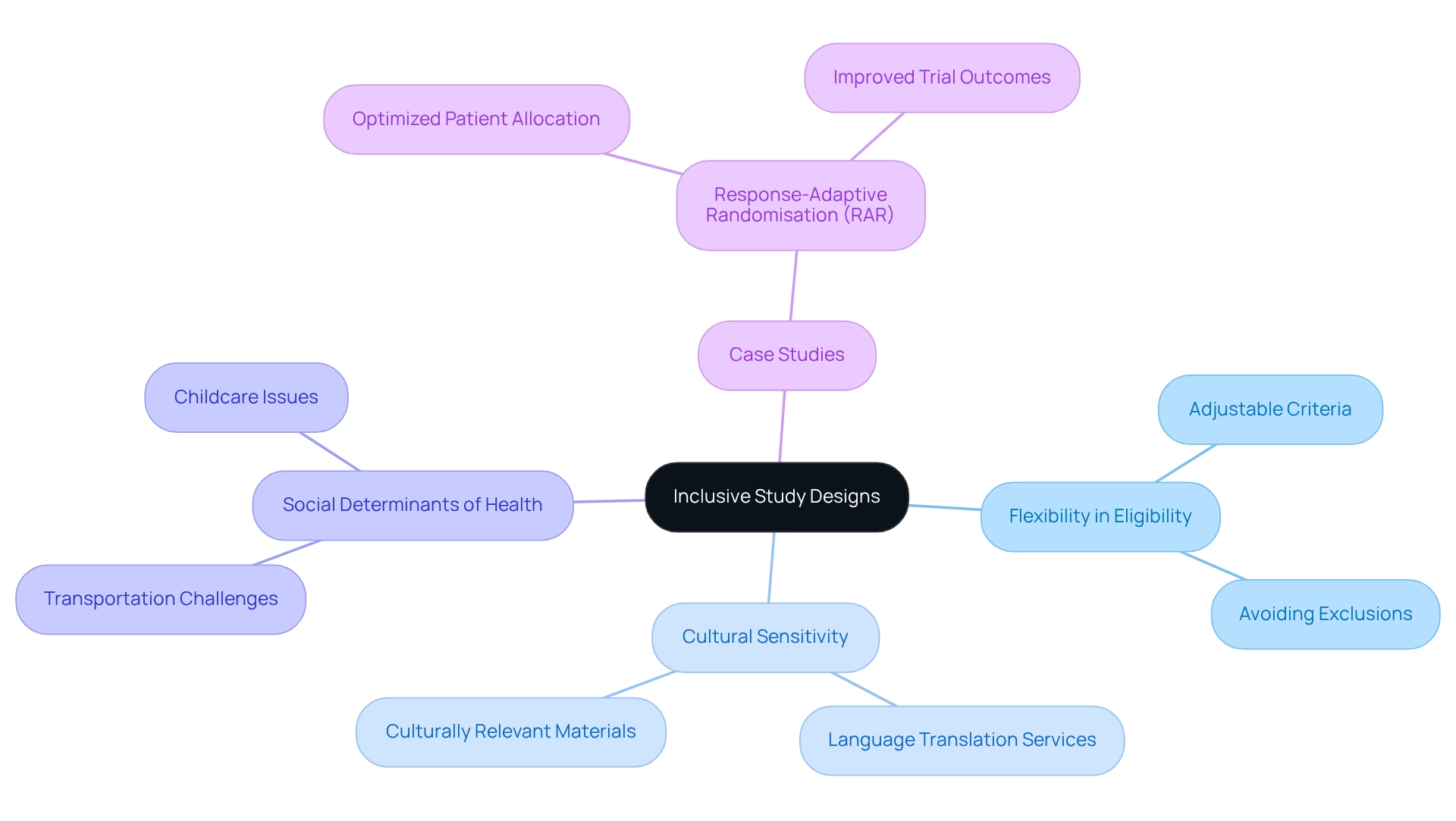
Design Inclusive Protocols and Study Designs
Inclusive study designs must prioritize the unique needs of diverse populations from the outset. This involves crafting protocols that incorporate flexibility in eligibility criteria to avoid inadvertently excluding specific groups. For instance, integrating language translation services and culturally sensitive materials can significantly enhance engagement with non-native speakers.
Furthermore, researchers should account for social determinants of health that may impact participation, such as transportation and childcare challenges. Statistics suggest that ophthalmology represented 3 percent of clinical studies globally from 2017 to 2022, emphasizing the significance of inclusivity in this area. By designing studies that are accessible and pertinent to a wide range of participants, researchers can gather more extensive information that accurately represents the effectiveness of medical interventions across different demographics.
The Response-Adaptive Randomisation (RAR) case study demonstrates how adaptive designs can enhance patient distribution to effective treatments, improving overall study outcomes. Moreover, as highlighted by Antidote, 'At Antidote, we are dedicated to offering reporting, statistics, and dynamic outreach strategies to ensure the studies we recruit for accurately represent every patient population.' This method not only enhances study results but also aligns with the increasing focus on patient diversity in Paraguay clinical trials.
Frequent mistakes in creating inclusive protocols involve inflexible eligibility standards that might leave out specific demographics, which can obstruct the overall efficacy of the study. By tackling these challenges, researchers can improve patient involvement and ultimately attain better study outcomes.
Engage Communities to Foster Participation in Trials
Involving groups is vital for improving participation in research trials. Researchers must actively engage local participants in both the planning and execution stages of studies to build trust and address any issues related to medical investigations. This can be effectively achieved through several strategies:
- Establishment of Community Advisory Boards: These boards provide valuable insights and feedback, ensuring that community perspectives are considered in the research process.
- Hosting Informational Sessions: Organizing events to educate residents about the advantages of research studies can demystify the process and encourage participation.
- Forming Partnerships with Local Organizations: Collaborating with local health clinics and advocacy groups helps reach diverse populations and establish trust within the area.
For instance, a prominent program in Paraguay successfully partnered with local health clinics to inform residents about the advantages of research studies, which significantly contributed to patient diversity in Paraguay clinical trials and resulted in a substantial rise in participation from various communities. As Scott Batey, a Professor in the School of Social Work at Tulane University, states, "Everyone has something important to share, and everyone has an equal opportunity to share it." This underscores the significance of valuing public input in the research process.
Moreover, bioaccess's extensive management services for research studies—including feasibility assessments, site selection, compliance evaluations, setup, project oversight, and reporting—are essential in ensuring that studies are conducted effectively and ethically. By prioritizing these elements, researchers can create a supportive environment that enhances community engagement.
Digital engagement approaches can also improve research infrastructure and boost public awareness. Studies indicate that patients prefer learning about research studies from their physicians (73%) or advocacy organizations (42%), yet only 32% reported that their doctors provided this information. By utilizing online platforms and social media, researchers can effectively connect with potential participants and offer valuable information about studies.
Prioritizing community engagement not only fosters a supportive environment but also enhances the overall success of clinical trials, ultimately contributing to more representative and effective research outcomes.
Conclusion
Patient diversity in clinical trials is not merely beneficial; it is essential for developing safe and effective treatments across all demographics. By ensuring that trials reflect the population's diversity, researchers gain critical insights into how various groups respond to treatments, thereby enhancing the validity of their findings. Addressing historical mistrust among underrepresented communities through transparent practices is imperative for fostering participation.
Effective recruitment strategies are pivotal in engaging diverse populations. Collaborating with community organizations, utilizing culturally relevant messaging, and offering flexible participation options can significantly enhance outreach and participation rates. As the clinical trial landscape evolves, prioritizing these strategies is crucial for rebuilding trust and promoting inclusivity, ultimately leading to improved health outcomes.
Designing inclusive study protocols further amplifies diversity. By incorporating flexible eligibility criteria and considering social determinants of health, researchers can create trials that are both accessible and relevant. Engaging communities through advisory boards and informational sessions fortifies the relationship between researchers and potential participants.
In summary, fostering patient diversity in clinical trials stands as both a regulatory necessity and a moral imperative that enhances the quality of research and promotes health equity. By emphasizing inclusivity, transparency, and community engagement, the clinical research community can drive advancements that benefit all populations, ensuring that medical breakthroughs are representative and effective for everyone.
Frequently Asked Questions
Why is patient diversity important in Paraguay clinical trials?
Patient diversity is essential in Paraguay clinical trials as it allows researchers to understand how different groups respond to treatments, which is crucial for developing safe and effective medical devices. It helps minimize biases and enhances the quality of medical research.
What factors influence drug metabolism and effectiveness in clinical trials?
Genetic, social, and cultural factors can significantly influence drug metabolism and effectiveness, highlighting the importance of including a wide range of participants in clinical trials.
What did the PLATINUM Diversity study reveal about patient diversity?
The PLATINUM Diversity study indicated that approximately 1 in 5 new molecular entities approved by the FDA have varying impacts on different racial and ethnic groups, underscoring the need for inclusive study designs.
How are regulatory organizations responding to the need for patient diversity in clinical trials?
Regulatory organizations are increasingly prioritizing patient diversity in clinical trials, recognizing it as vital for effective study design and execution.
What historical example illustrates skepticism towards medical institutions in communities of color?
The Tuskegee Syphilis Experiment serves as a historical case study that illustrates the lingering skepticism towards medical institutions within communities of color, emphasizing the need for transparency and ethical practices in research.
What steps can be taken to promote patient diversity in clinical trials?
Steps to promote patient diversity include engaging with diverse communities to understand their concerns, implementing culturally sensitive recruitment strategies, ensuring clarity about the research process and benefits, and providing education about the significance of diversity in health-related research.
How does patient diversity contribute to health outcomes?
Patient diversity enhances the reliability of research findings and contributes to the development of medical devices that are effective across diverse populations, ultimately leading to improved health outcomes for all.




