Overview
Patient recruitment for clinical trials in Argentina can be significantly enhanced through a comprehensive understanding of local regulations, cultural factors, and innovative recruitment strategies. This approach captures the attention of stakeholders by emphasizing the importance of leveraging local insights and building strong relationships.
Moreover, utilizing digital tools can improve recruitment outcomes, addressing the unique challenges and dynamics of the Argentine clinical research landscape. By reinforcing the significance of collaboration and the expertise required in this field, we can generate a desire for effective recruitment solutions.
Ultimately, action must be taken to implement these strategies for successful clinical trial recruitment.
Introduction
In the rapidly evolving field of clinical research, Argentina emerges as a distinctive landscape shaped by its regulatory framework and cultural nuances. With the National Administration of Drugs, Food and Medical Technology (ANMAT) at the helm, grasping the intricacies of trial registration and ethical considerations is paramount for research teams. As the number of registered clinical studies continues to soar, effective recruitment strategies become essential to navigate the complexities of patient engagement in this diverse nation.
By leveraging local insights and fostering strong relationships with stakeholders, the path to successful clinical trials in Argentina is paved with opportunities for innovation and collaboration. Embracing these strategies enables research teams to enhance participant recruitment while contributing to the advancement of medical research and technologies that benefit communities worldwide.
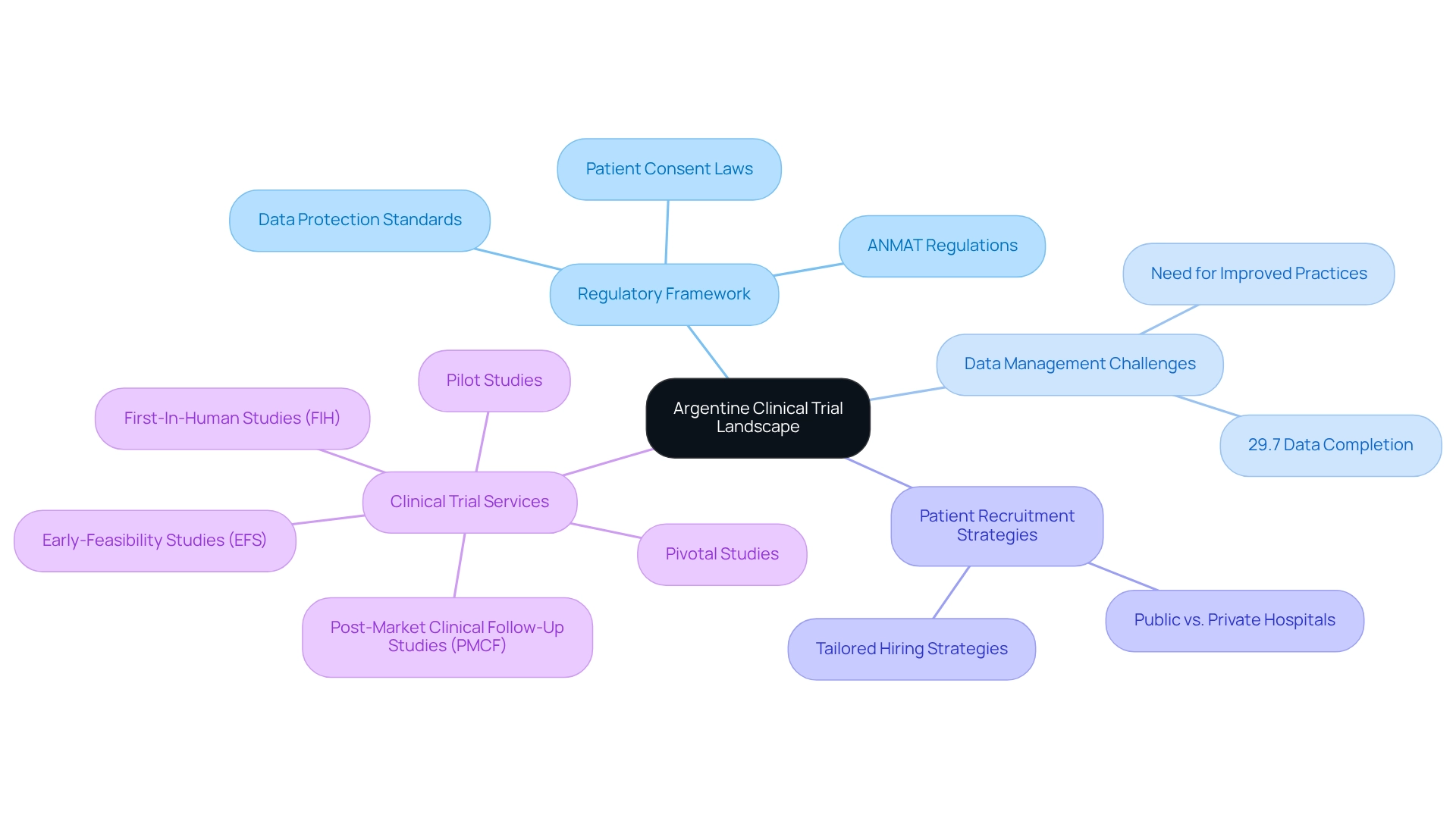
Understand the Argentine Clinical Trial Landscape
Argentina's research environment is defined by a comprehensive regulatory framework overseen by the National Administration of Drugs, Food and Medical Technology (ANMAT). A thorough understanding of specific regulations, including the research registration process and ethical considerations, is crucial for any clinical research team. Trials must comply with local laws regarding patient consent and data protection, which can vary significantly from those in other regions. This knowledge not only ensures compliance but also enhances the study's credibility, making it more attractive to potential participants.
As of 2025, statistics indicate that only 29.7% of research records have completed the essential data fields related to methods. This figure underscores the urgent need for improved data management practices, particularly as the research landscape in Argentina evolves with a notable increase in Phase 3 studies. Such trends necessitate tailored hiring strategies that align with the specific types of studies being conducted.
Moreover, challenges in conducting medical studies in public hospitals, often hindered by bureaucracy and delays, highlight the importance for research groups to consider private hospitals, which typically operate more efficiently. Understanding these regulatory nuances and the current research study landscape is essential for effective patient recruitment for trials in Argentina. By ensuring that strategies are relevant and compliant with local standards, research groups can significantly enhance their chances of successful patient recruitment and retention in studies.
As Julio G. Martinez-Clark, CEO of bioaccess®, asserts, "Argentina has made significant contributions to medical device innovations, with several notable inventions that have had a global impact." This context emphasizes the critical role of research studies in advancing medical technologies. Furthermore, grasping the ANMAT research registration process is vital for compliance, as it lays the groundwork for effective patient recruitment for trials in Argentina.
By avoiding common pitfalls in hiring practices, research groups can navigate the complexities of the Argentine medical study environment more adeptly.
With bioaccess®'s expertise in comprehensive clinical trial management services, including:
- Early-Feasibility Studies (EFS)
- First-In-Human Studies (FIH)
- Pilot Studies
- Pivotal Studies
- Post-Market Clinical Follow-Up Studies (PMCF)
research teams can refine their recruitment strategies and ensure adherence to data protection standards.
Leverage Local Insights for Effective Recruitment
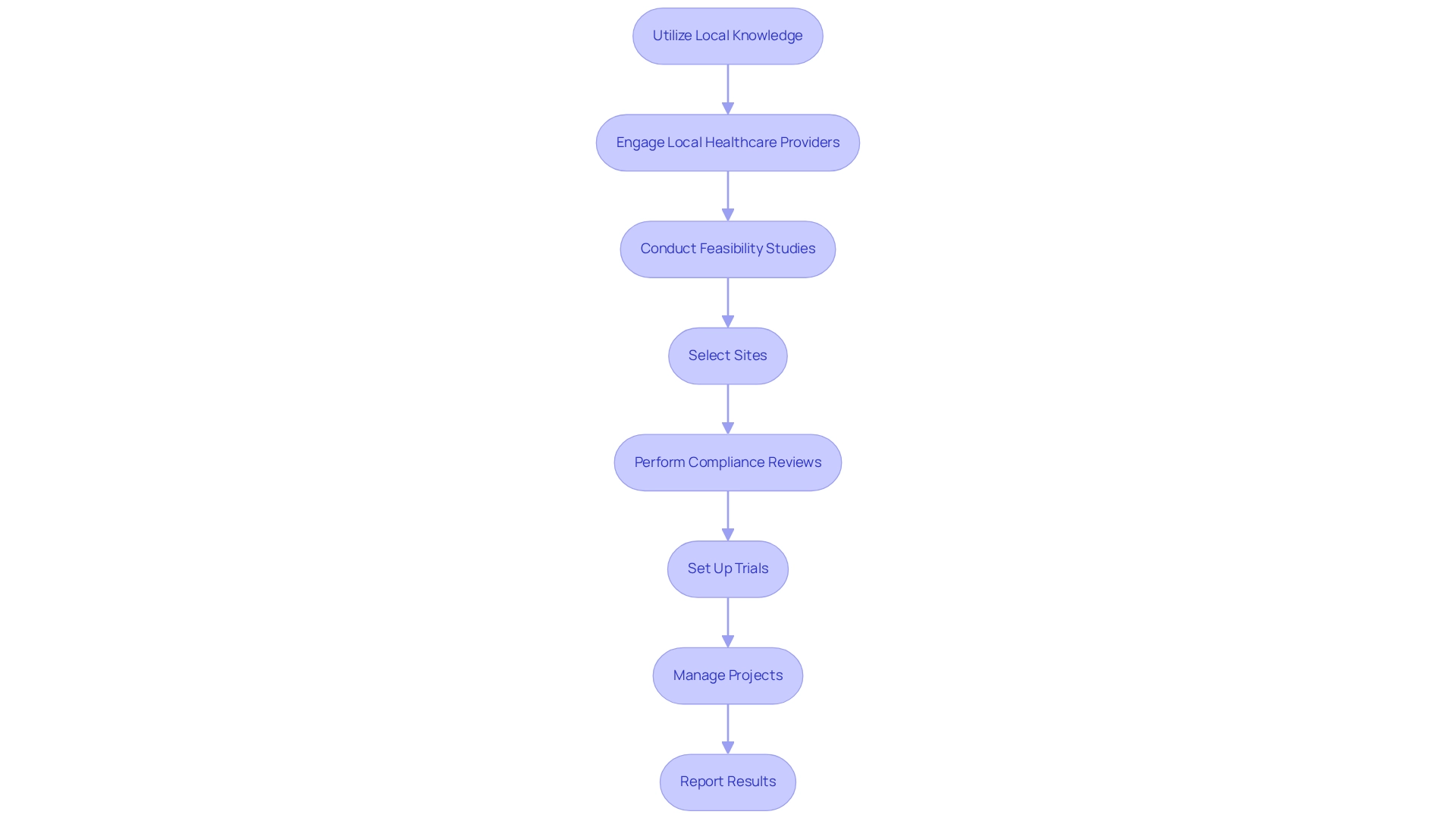
Utilizing local knowledge is essential for comprehending the cultural, social, and economic factors that affect patient recruitment for trials in Argentina. The country's diverse population exhibits a wide range of health beliefs and practices, making it crucial to engage local healthcare providers who can navigate these nuances. Their participation cultivates trust and substantially enhances recruitment outcomes.
In 2023, the count of registered research studies reached 453,803, underscoring the competitive environment for medical experiments in Argentina and the significance of patient recruitment for trials. Comprehensive clinical trial management services, such as those offered by bioaccess, enhance these efforts through:
- Feasibility studies
- Site selection
- Compliance reviews
- Trial setup
- Project management
- Reporting
These services ensure that local regulations and cultural contexts are respected. Moreover, employing community health workers can bolster patient recruitment by engaging with potential participants through relatable and culturally aware communication.
As one local saying goes, "In Argentina, cooking is a way to bring people together," illustrating the importance of community and trust in attracting patients. Customizing outreach messages to address specific local health issues and emphasizing the advantages of involvement—such as access to innovative treatments—can significantly improve patient recruitment by resonating more effectively with potential participants. Furthermore, the case study titled "Future Perspectives on Clinical Trials in Latin America" highlights the necessity for enhanced infrastructure and collaboration among stakeholders, which is essential for optimizing enrollment efforts.
By incorporating these local insights and utilizing comprehensive study management services into patient recruitment for trials in Argentina, research teams can develop a more inclusive and efficient enrollment process, ultimately enhancing participation rates in studies.
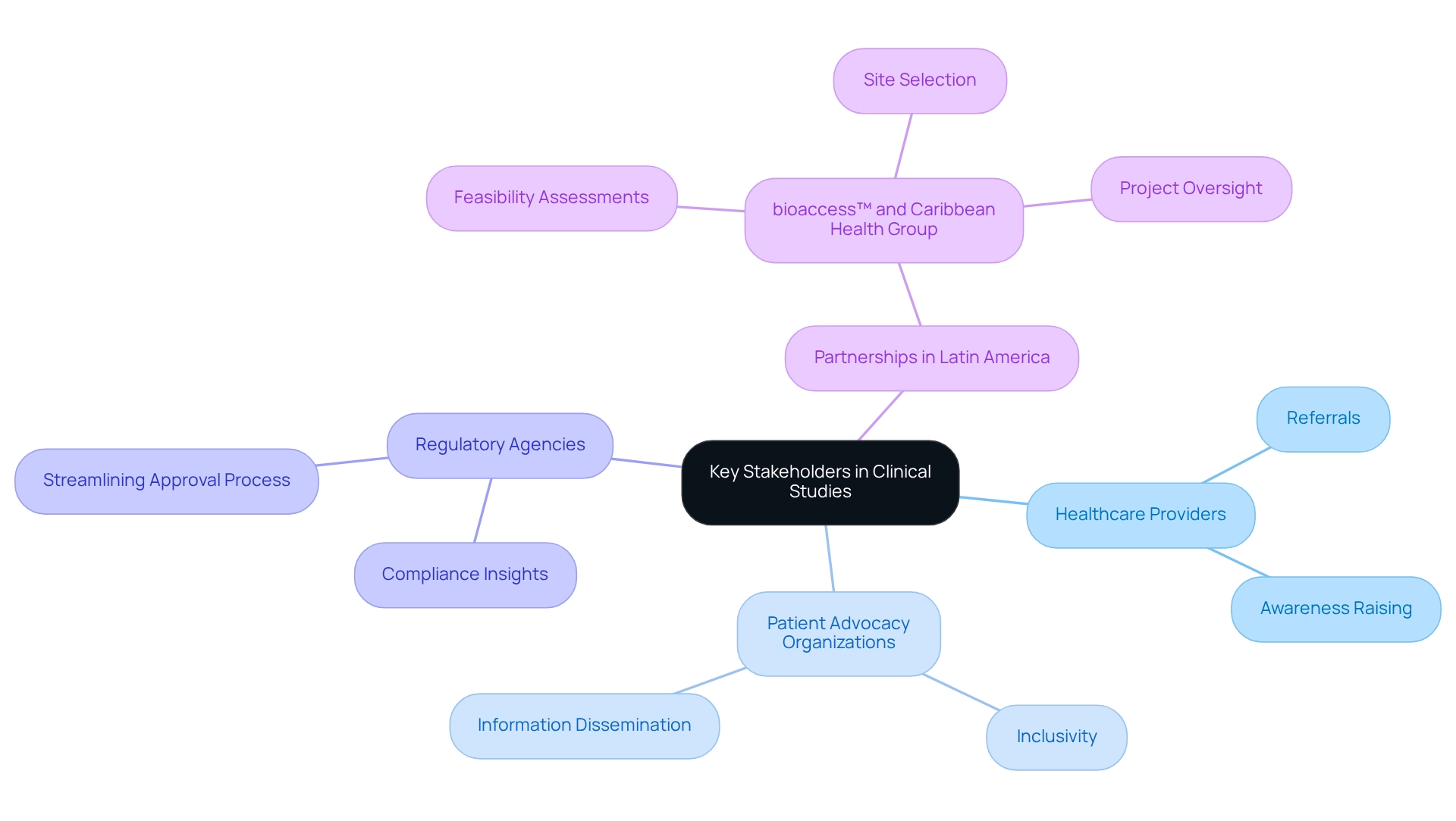
Build Strong Relationships with Key Stakeholders
Establishing strong connections with key stakeholders is essential for enhancing patient enrollment in clinical studies. Collaborating with local healthcare providers not only facilitates referrals but also raises awareness of studies among potential participants. In Argentina, data from the Clinical and Translational Science Award (CTSA) program reveal that patient recruitment for trials is significantly influenced by healthcare provider referrals, underscoring the necessity for robust partnerships.
Engagement with patient advocacy organizations further strengthens recruitment initiatives by disseminating study information to diverse patient communities, ensuring inclusivity and representation.
Moreover, maintaining consistent communication with regulatory agencies provides critical insights into compliance requirements, thereby streamlining the approval process for studies. A notable case study illustrates how a research team collaborated with a qualitative research group to enhance methodological rigor, ultimately preserving the objectivity and reliability of their findings. This collaboration emphasizes the importance of stakeholder engagement in ensuring high-quality research outcomes.
In Colombia, the partnership between bioaccess™ and Caribbean Health Group aims to position Barranquilla as a leading hub for medical studies in Latin America, supported by Colombia's Minister of Health. This initiative not only elevates the visibility of medical studies but also fosters a supportive environment for patient recruitment. bioaccess™ offers comprehensive management services for studies, encompassing feasibility assessments, site selection, compliance evaluations, study setup, import permits, project oversight, and reporting.
As Dr. Beth Garner, Chief Scientific Officer of Ferring Pharmaceuticals US, articulated, "It’s so thrilling to offer research studies to the public." Furthermore, media coverage by Clinical Leader has highlighted the significance of research studies in Latin America, further amplifying awareness and participation. By nurturing these connections, research teams can cultivate a supportive environment that not only aids in attracting participants but also enhances the overall success of the study, ultimately contributing to the development of life-saving therapies and improved healthcare outcomes.
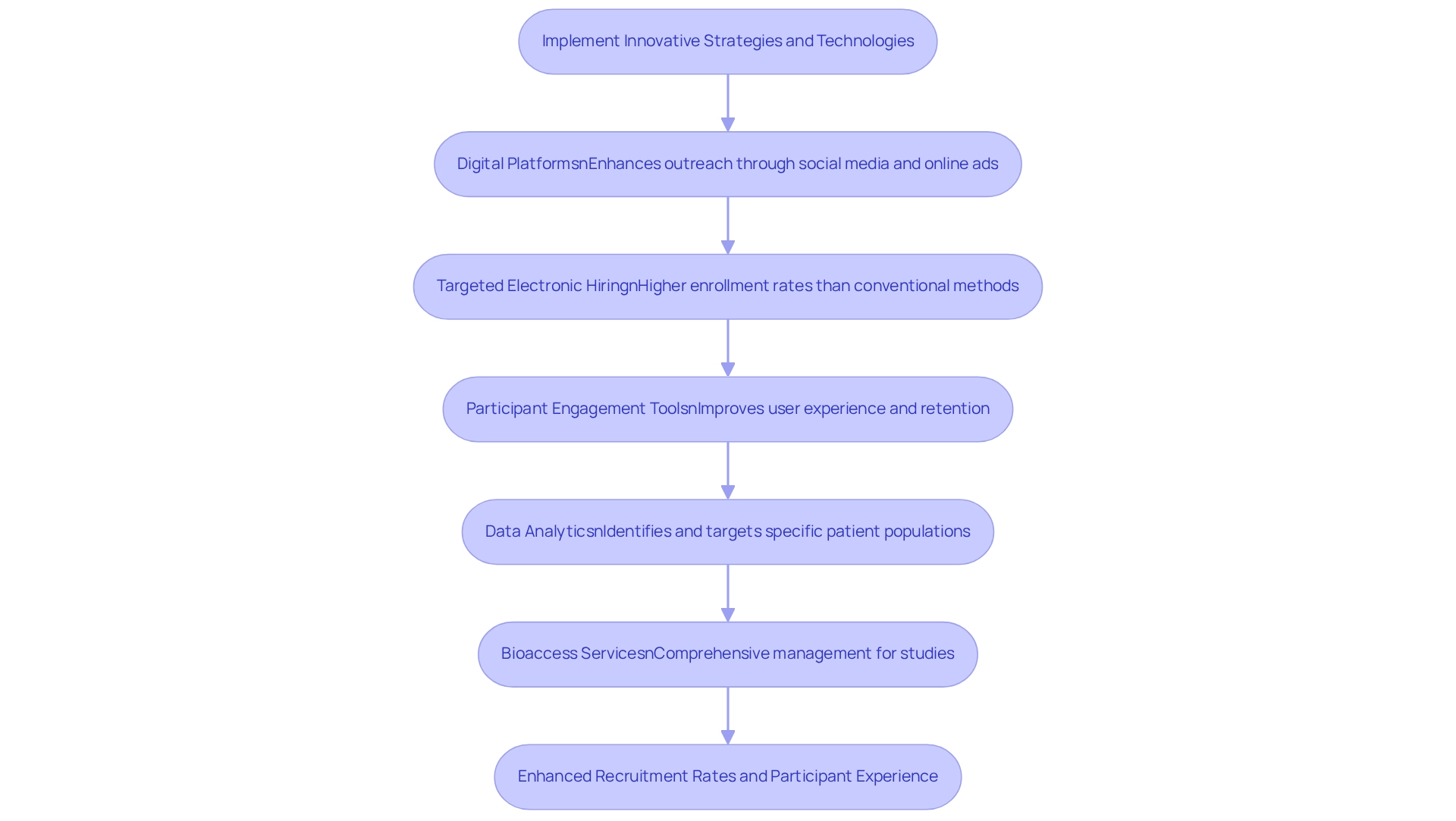
Implement Innovative Strategies and Technologies
Implementing innovative strategies and technologies is crucial for patient recruitment in clinical trials across Argentina. Digital platforms, including social media and targeted online advertising, significantly enhance outreach and support patient recruitment by attracting a diverse participant pool. Studies indicate that targeted electronic hiring techniques yield higher enrollment rates compared to conventional methods, such as banner advertisements. As noted by Gonzalez, S., "Greater enrollment occurred through the targeted electronic recruitment method than through banner ads." Furthermore, participant engagement tools, such as mobile applications that provide study information and updates, improve user experience and retention. Virtual screening procedures facilitate enrollment, allowing potential participants to engage in studies from the comfort of their homes.
A notable example of the efficiency of digital tools is the text messaging system that identified individuals for an acute stroke trial, achieving a median time of 62 minutes from admission to the text message. This showcases how such technologies can accelerate participant enrollment. Leveraging data analytics emerges as another key strategy, enabling researchers to identify and target specific patient populations more effectively. This approach simplifies hiring efforts while ensuring they are both effective and significant.
Moreover, bioaccess™ offers extensive management services for studies, including:
- Feasibility assessments
- Site selection
- Compliance evaluations
- Setup
- Import permits
- Project oversight
- Reporting
These services are essential for patient recruitment in trials across Argentina. The partnership between bioaccess™ and Caribbean Health Group aims to position Barranquilla as a prominent hub for medical research in Latin America, supported by Colombia's Minister of Health. This initiative not only fosters international collaboration but also contributes to local economic growth through job creation and healthcare improvement. Additionally, GlobalCare Clinical Trials' collaboration with bioaccess™ has resulted in over a 50% reduction in enrollment time and impressive 95% retention rates, demonstrating the effectiveness of these strategies.
As the landscape of clinical trials continues to evolve, embracing these innovative methods will not only enhance recruitment rates but also improve the overall participant experience, ultimately contributing to the success of clinical research initiatives. Insights from the case study titled "Guidance for Digital Tools in Clinical Studies" emphasize the importance of compliance with ethical and regulatory standards when implementing these digital tools, which is vital for the target audience.
Conclusion
Successfully navigating Argentina's clinical trial landscape hinges on understanding the regulatory framework set by ANMAT. With only 29.7% of trial records fully completed, enhancing data management and recruitment strategies is imperative. Recognizing the benefits of private hospitals over public ones can further improve patient engagement and recruitment efforts.
Leveraging local insights is essential, as Argentina's diverse population has varying health beliefs. Engaging local healthcare providers and community health workers builds trust, making recruitment more effective. Tailoring messages to resonate with specific health concerns and highlighting participation benefits can significantly boost engagement in this competitive environment of over 453,803 registered clinical studies.
Establishing strong relationships with key stakeholders is crucial for recruitment success. Collaborating with healthcare providers and patient advocacy groups increases trial awareness and facilitates referrals. Regular communication with regulatory bodies can streamline the approval process and enhance research quality.
Moreover, implementing innovative strategies and technologies is vital for effective recruitment. Utilizing digital platforms and data analytics enables targeted outreach, while patient engagement tools enhance the participant experience. By adopting these strategies, clinical research teams can improve recruitment rates and contribute to successful trials that advance medical knowledge and technology in Argentina.
Frequently Asked Questions
What is the regulatory framework for clinical research in Argentina?
Argentina's clinical research environment is governed by a comprehensive regulatory framework overseen by the National Administration of Drugs, Food and Medical Technology (ANMAT). Understanding specific regulations, including the research registration process and ethical considerations, is essential for clinical research teams.
Why is understanding local laws important for clinical trials in Argentina?
Understanding local laws regarding patient consent and data protection is crucial, as these can differ significantly from those in other regions. This knowledge ensures compliance and enhances the credibility of the study, making it more appealing to potential participants.
What is the current state of data management in Argentine research?
As of 2025, only 29.7% of research records in Argentina have completed the essential data fields related to methods. This highlights the need for improved data management practices, especially as the number of Phase 3 studies increases.
What challenges do research groups face when conducting studies in public hospitals in Argentina?
Conducting medical studies in public hospitals is often hindered by bureaucracy and delays. As a result, research groups may find it more efficient to consider conducting studies in private hospitals.
How can research groups improve patient recruitment for trials in Argentina?
Research groups can enhance patient recruitment by understanding regulatory nuances, ensuring compliance with local standards, and adopting relevant strategies tailored to the specific types of studies being conducted.
What role has Argentina played in medical device innovations?
Argentina has made significant contributions to medical device innovations, with several notable inventions that have had a global impact, emphasizing the importance of research studies in advancing medical technologies.
What types of clinical trial management services does bioaccess® provide?
Bioaccess® offers comprehensive clinical trial management services, including Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF).
How can research teams navigate the complexities of the Argentine medical study environment?
By avoiding common pitfalls in hiring practices and understanding the regulatory landscape, research groups can navigate the complexities of the Argentine medical study environment more effectively.




