Introduction
Embarking on clinical trials in Latin America comes with its own set of challenges and opportunities. From addressing the underrepresentation of minority groups in research to overcoming access barriers and cultural nuances, there are several factors to consider for successful patient recruitment. Additionally, the rapidly evolving healthcare landscape in Latin America, along with Brazil's potential to influence global health policies, emphasizes the need for regionally relevant and globally impactful trials.
Building effective advertising strategies, understanding the target audience, utilizing digital recruitment campaigns, and engaging with patient communities and advocacy groups are essential for enhancing recruitment efforts. Furthermore, ensuring site engagement and support, addressing health equity and diversity, tracking progress and metrics, and leveraging technology are key components in streamlining patient recruitment strategies. Lastly, prioritizing informed consent and patient privacy is crucial in upholding ethical research practices.
By adopting these approaches, clinical trials in Latin America can foster inclusivity, trust, and patient-centricity while advancing medical science and improving healthcare outcomes.
Determining Feasibility and Study Requirements
Starting trials in Latin America requires a thorough understanding of the region's unique challenges and opportunities. Key considerations for the feasibility of patient recruitment include not only sample sizes, but also the specific inclusion and exclusion criteria that reflect the diverse population. The underrepresentation of minority groups in medical research is a significant concern, as it can skew the data and lead to less effective treatments for these communities. Addressing historical mistrust, such as the lingering impact of the Tuskegee Syphilis Study, and overcoming access barriers like healthcare availability, financial constraints, language differences, and cultural nuances are critical for successful enrollment.
Moreover, the swiftly developing healthcare scenario in Latin America, demonstrated by Brazil's prominence in the G20 and its capacity to shape international health policies, emphasizes the requirement for studies that are both regionally pertinent and globally influential. The incorporation of experiments with medical practice, as addressed at the JAMA Summit, is crucial to guarantee that test outcomes are relevant in real-life environments.
Clinical experiments play a role in groundbreaking healthcare progress, and engaging in such experiments provides individuals the chance to contribute to this significant change. By utilizing the growing medical and scientific expertise in Latin America and addressing the methodological requirements with an evidence-based framework, it is possible to lead to more effective and inclusive experiments. By doing so, we not only adhere to ethical and scientific standards but also honor the collaborative spirit that drives progress in medical research.
Building an Effective Advertising Strategy
Understanding the cultural and linguistic nuances is crucial for the success of clinical trial recruitment in South America. For instance, educational initiatives addressing societal taboos, similar to the Anariá project in Brazil, can be instrumental. This project worked within the public system to provide cervical cancer testing and care, reaching nearly 9,000 women and conducting over 25,000 consultations, thus bridging the gap in access to specialized healthcare. Such efforts not only facilitate early diagnosis and treatment but also build trust within communities, which is essential for successful patient recruitment.
Moreover, as Brazil takes the lead with the G20 presidency in 2024, there is a unique opportunity to spotlight health issues and solutions pertinent to Latin America, showcasing the region's growing medical expertise. This is a favorable moment for experiments to utilize the momentum and improve recruitment strategies.
Recruitment campaigns should also resonate with the target demographic, which means employing tailored language and culturally relevant messaging. As highlighted by NIH officials, communication is crucial for inclusive research, ensuring that diverse populations understand the extent and advantages of participating in medical experiments.
Furthermore, providing patient-centric support, such as the Navigator service, can significantly enhance recruitment and retention. This service provides a 'human touch' through direct communication with trained professionals, showcasing how valued and respected participants are in their research experience. In South America, this method could be adjusted to offer in-country assistance from indigenous speakers, aligning with the regional context.
Lastly, leveraging social media with initiatives like the #SaludTues Tweetchat can engage and inform Latino communities about the significance of participating in research studies. This conversation can foster awareness and motivate potential volunteers to partake in research that could lead to groundbreaking health and medical discoveries.
Defining and Understanding the Target Audience
Efficient recruitment strategies are crucial for the success of studies, particularly in the diverse and dynamic area of Latin America. A nuanced approach is necessary to comprehend the demographic, geographic, and socio-economic factors that impact populations in need of medical care. For example, linguistic diversity poses a significant challenge in the region. In the United States alone, nearly 68 million people report speaking a language other than English at home, with a considerable number speaking Spanish. This fact highlights the significance of language considerations in participant recruitment and the necessity to guarantee that materials for medical research are not only precisely translated but also culturally customized to connect with the intended viewers.
Additionally, focusing on the needs of individuals is essential in designing clinical experiments, which includes actively engaging individuals in the planning process and guaranteeing that the experiment information is accessible and understandable. This approach fosters diversity, equity, and inclusion, ensuring that trials include participants from various backgrounds and experiences, reflecting the true spectrum of the disease being studied.
Identifying potential participants involves leveraging existing patient databases and conducting market research. Focusing on areas with high disease incidence and prevalence can streamline the recruitment process. Collaborating with local partners who understand the cultural and regulatory nuances can further enhance recruitment efforts. For example, navigating the specific consent requirements for vulnerable populations or investigational product definitions in South America requires in-depth knowledge of local regulations.
The QuEST LAC network exemplifies the drive to enhance healthcare quality in America. By fostering research capacity and sharing high-quality health system sciences, this network contributes to better health outcomes and builds trust within communities. Such cooperative endeavors can be crucial in advancing recruitment strategies in the region.
In a wider perspective, Brazil's upcoming G20 presidency offers a chance to showcase the contribution of Latin American countries to global health and medical research. The region's unique challenges, from healthcare worker shortages to climate change impacts, are a microcosm of global public health issues. Tackling these obstacles through improved recruitment for medical experiments can play a crucial role in promoting health equity and the progress of medical science.
Utilizing Digital Recruitment Campaigns
Utilizing digital recruitment campaigns presents a transformative avenue for engaging potential research participants in Latin America. These strategies leverage the power of targeted online advertisements, search engine optimization, and extensive social media outreach to bridge the gap between research and participation. Digital tools not only offer cost-effectiveness and an expansive reach but also provide real-time insights into the recruitment process, enhancing the efficiency of enrollment.
The integration of digital platforms addresses important challenges such as the underrepresentation of diverse populations in medical research. By tapping into a broader demographic through online channels, clinical trials can ensure a more inclusive approach that reflects a wider range of genetic backgrounds and medical histories, thereby improving the relevance and applicability of research outcomes to various groups.
Moreover, digital approaches can help overcome historical mistrust and access barriers. For instance, in Uruguay, a successful initiative to improve women's participation in cervical cancer screening involved a two-step investment in digital health services. By centralizing health information and creating an accessible online system for scheduling Papanicolaou Test appointments, the project demonstrated the potential of digital platforms to enhance preventive healthcare behaviors and potentially reduce mortality rates.
In the context of Latin America, where disparities in access to effective and timely care for rare diseases persist, the adoption of digital recruitment methods could be particularly impactful. With almost half of people expressing a preference for digital interactions in healthcare, and a significant portion interested in online payment options, there is a clear demand for more flexible and user-friendly digital experiences.
As digital therapeutics emerge as a promising field for managing chronic diseases, their potential for reducing patient costs underscores the value of digital innovation in healthcare. This is particularly relevant as the region deals with the requirement for more efficient and fair health service delivery, as emphasized by health policy experts and those experienced in implementing digital projects within medical settings.
In conclusion, recruitment campaigns in Latin America offer an opportunity to address long-standing issues in participation in research. By embracing digital tools, researchers can promote a more inclusive, trustworthy, and patient-centric approach to recruitment for medical experiments.
Engaging with Patient Communities and Advocacy Groups
Establishing collaborations with communities and support organizations is a fundamental aspect in improving participant recruitment for research studies, especially in Latin America. These collaborations are essential in not only expanding the pool of potential experiment participants but also in ensuring that the demographics of the participants reflect the diversity needed for strong and inclusive research outcomes.
Interaction with groups of individuals undergoing medical treatment offers a channel for reaching those who may be motivated and committed to taking part in medical research, thereby tackling the crucial problem of underrepresentation of minority groups in medical experiments. Utilizing the influence of organizations representing individuals seeking medical assistance, which have experienced substantial growth in the previous ten years, can aid in closing the divide in participation in experiments by offering reliable information and assistance to prospective participants, nurturing an approach to research design that prioritizes the needs of patients.
Informing prospective participants about the real-life consequences of clinical experiments is crucial. This could be achieved through organizing educational events that communicate the value and potential impact of their participation in a manner that is both balanced and comprehensible. It's crucial to tackle past distrust and obstacles to reach out with relevant information that connects with the individual's encounter and is mindful of cultural subtleties.
The engagement of individuals representing the individuals undergoing medical treatment in the design and execution of research studies can be a game-changing approach. As pointed out by industry professionals, actively incorporating the views of individuals receiving medical care guarantees that their perspectives and requirements are prioritized in the planning of experiments. Furthermore, this comprehensive approach can result in the creation of more patient-centered studies, thereby improving the quality and relevance of the research.
To illustrate the impact of these strategies, consider the Mamás del Río program in Peru, which adapted community healthcare initiatives to a post-pandemic setting. Such real-life examples demonstrate how policy changes informed by patient-centric research can lead to systemic improvements in healthcare systems.
In brief, through establishing robust communication channels and involving communities of individuals receiving medical care in the core of research processes, investigators can not only enhance enrollment rates but also improve the integrity and pertinence of their studies, ultimately progressing towards a more healthcare ecosystem centered around individuals.
Enhancing Site Engagement and Support
To maximize patient recruitment in Latin America, clinical study sites must be fully engaged and supported. Highlighting the importance of providing extensive training and resources to site staff is crucial, ensuring that they are equipped with the knowledge and tools necessary to effectively oversee experiments and facilitate participant enrollment. Collaboration between study coordinators and investigators is crucial, fostering a team environment that can address challenges dynamically and maintain the momentum of the research.
Leveraging technology and digital advancements is also critical. As the Vice President of Digital Offerings at RWS, Daniel J Herron, emphasizes the importance of patient-centric approaches, stating that involving patients in the design of experiments and delivering easily understandable information is fundamental. This aligns with the need for diversity, equity, and inclusion in studies, ensuring a broad representation of participants with varying backgrounds and experiences.
Moreover, the global shift towards decentralized research studies (DCTs) emphasizes the importance of engaging participants where they are, which is particularly relevant in the diverse and dispersed populations of Latin America. This method has experienced a compound annual growth rate of 30.1% from 2021 to 2026, indicating a swift embrace of approaches that bring experiments to the individual's residence, thus decreasing obstacles to engagement.
Astrum, a merger of several CROs, including BlueClinical, showcases the potential of extended services and capabilities in clinical studies. Luis Almeida, Managing Director of BlueClinical, highlights the strategic benefits of this merger, illustrating the opportunities for career development and enhanced service offerings that can arise from such collaborations.
In general, the effectiveness of participant enrollment approaches in the region of America relies on the incorporation of these essential components: extensive training for site personnel, cooperative frameworks, technological integration for efficient site procedures, and the implementation of participant-focused and distributed examination models to improve the participant experience.
Addressing Health Equity and Diversity in Recruitment
Clinical experiments in Latin America must prioritize health equity and diversity to ensure that research outcomes are applicable and beneficial across all segments of the population. Acknowledging cultural, linguistic, and socioeconomic factors is critical when engaging participants from this region. An all-encompassing approach to research is not only a matter of ethical research practice but also a cornerstone for achieving personalized medicine for diverse patient populations.
The lack of sufficient representation from specific demographic groups, including those characterized by age, ethnicity, gender identity, or socioeconomic status, can restrict the applicability of research findings. For example, ethnic minorities, although making up 40% of certain populations, may only account for 2-15% of participants in medical studies. These disparities in research participation can introduce biases, potentially resulting in healthcare treatments that fail to address the needs of a significant portion of the population.
Integrating diversity and inclusion into medical studies starts with establishing trust within communities, especially those that have traditionally been excluded or marginalized in research. As noted by a leader of a National Cancer Institute-designated cancer center, it is essential to engage with groups early on, even before they become eligible for study participation. Previous breaches of trust in medical research underscore the importance of establishing credibility and fostering relationships in areas of prevention and screening.
Recent advancements in technology, such as the utilization of artificial intelligence, are streamlining the process of identifying and recruiting targeted populations. This encompasses a range of individuals from different backgrounds, including people of color, women, older adults, and Hispanic and Latino groups. By leveraging data from electronic health records, health systems, and health tech companies, pharmaceutical organizations can develop comprehensive diversity plans that meet the latest FDA guidelines.
Despite progress in recent years, the challenge persists: over 40% of the U.S. population consists of racial and ethnic minorities, yet clinical trial participation from these groups often falls between a mere 5 to 10%. This disparity emphasizes a crucial lack of understanding concerning the effectiveness and safety of medical treatments for individuals from minority groups, which poses a threat to individual well-being and treatment results. In South America, dealing with these disparities is especially important because of the diverse population demographics and the region's unique healthcare challenges.
Tracking Progress and Metrics for Recruitment Success
To enhance recruitment strategies in clinical studies within the region, it is crucial to closely monitor important metrics and indicators. These include enrollment rates which reflect the speed and efficiency of patient recruitment, screening and eligibility criteria ensuring the right participants are selected, retention rates to gauge participant engagement throughout the study, and participant satisfaction which serves as feedback on the study experience. Leveraging data analytics and reporting tools for thorough analysis of recruitment data facilitates informed decision-making, enabling adjustments to strategies in real-time to improve outcomes. For instance, employing gamification can enhance user engagement, as seen in applications that have successfully integrated this approach to maintain user involvement. Similarly, the concept of crowdsourcing can provide valuable insights into participant interactions and characteristics, as demonstrated in projects utilizing this method to gather significant user data. In Latin America, addressing barriers to participation such as linguistic and cultural differences is also crucial. This is proven by collaborations like the partnership between Dana-Farber Cancer Institute and OncoclÃnicas, which indicate a dedication to high-quality, patient-centered care, reflecting the significance of international standards and protocols in clinical experiments. Emphasizing diversity, equity, and inclusion (DEI) in trial participation ensures a comprehensive understanding of treatment effects across different demographics, thus enhancing the quality and applicability of research conclusions. As underscored by the Quest LAC network's efforts, quality health systems are fundamental for better health outcomes, with research indicating that nearly 8 million deaths in LMICs are due to poor-quality healthcare despite service access. Therefore, careful monitoring of these metrics is not only a procedural requirement but a foundation for advancing healthcare quality and patient trust in America.
Case Study: Successful Patient Recruitment Strategies in Action
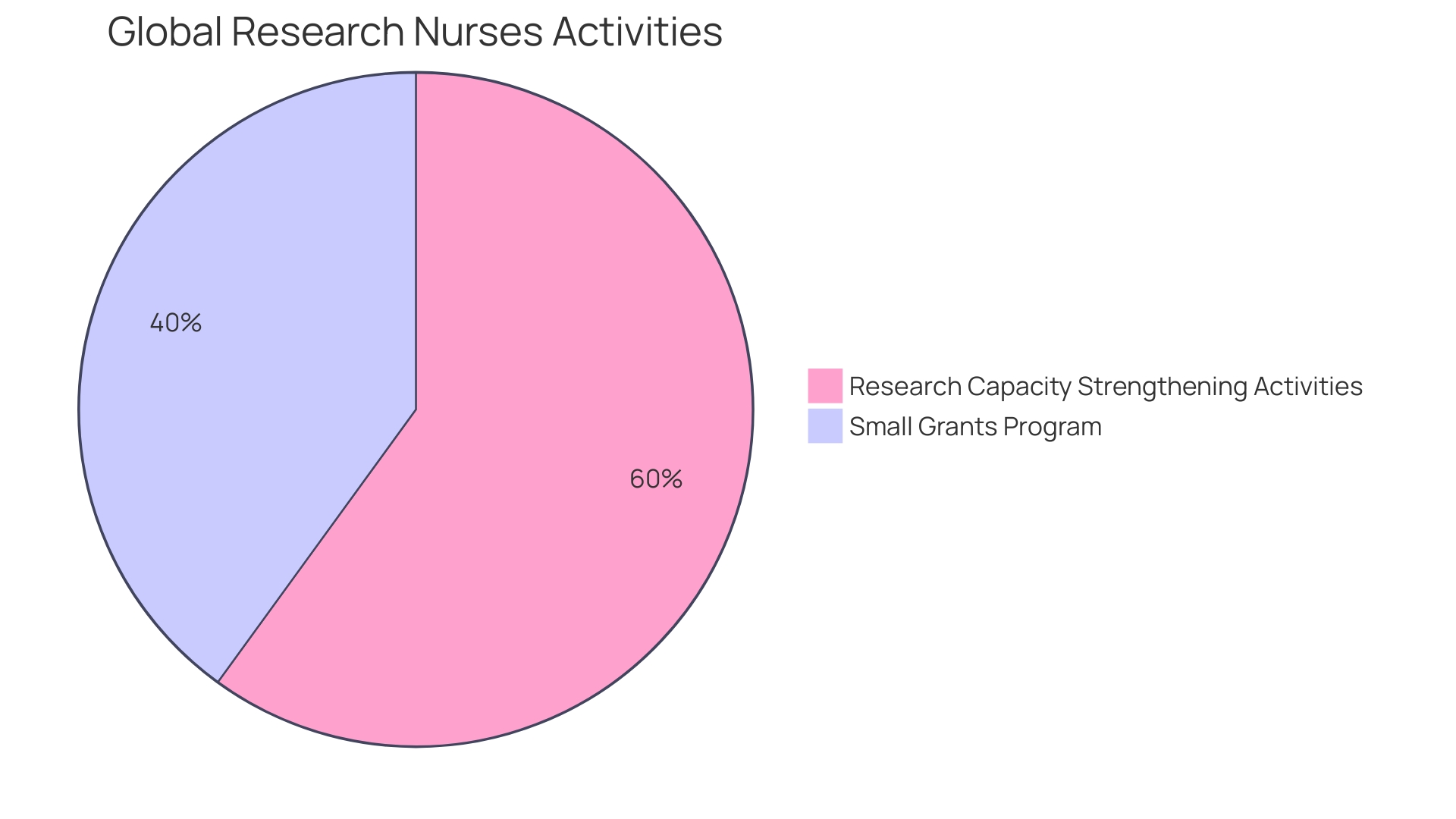
Arancha de La Horra, the project lead for Global Research Nurses, a group under The Global Health Network, has been at the forefront of fostering research involvement among nurses and midwives in America. Through workshops and small grants, her team has empowered healthcare professionals in Ethiopia and beyond to enhance their qualitative research skills. This initiative is divided into modules, one of which focuses on developing research proposals, equipping nurses with the tools to lead in research. Brazil, as the upcoming G20 president, positions America to address public health issues, leveraging its medical expertise and diverse cultures to strengthen global health leadership. In the face of public health trials like the COVID-19 pandemic, South America has demonstrated resilience by building local capacities and health cooperation. Meanwhile, Kati, a manager at Economist Impact, underscores the need for research and policy analysis to tackle disparities in rare disease care in the region. The Quality Evidence for The Transformation of Health Systems for America and the Caribbean network exemplifies regional collaboration to enhance healthcare quality. These instances demonstrate the active involvement of countries in South America in enhancing health results and emphasize the area as a center for progressing research and recruitment strategies.
Common Challenges and Solutions in Patient Recruitment
Enlisting individuals for experiments in Latin America presents distinctive difficulties that need to be tactically addressed to guarantee favorable results. One of the main obstacles is the restricted consciousness and comprehension of clinical experiments among potential participants. This lack of knowledge can be compounded by language barriers, making it imperative to provide clear and understandable information in multiple languages. Moreover, operational challenges like distant transportation to examination locations and regular appointments may amplify the strain on individuals, possibly resulting in reduced enrollment and increased attrition levels.
To overcome these hurdles, a patient-centric approach has proven to be effective. Including individuals in the planning and design of experiments guarantees their viewpoints and requirements are at the core of the procedure. Emphasizing diversity, equity, and inclusion is also critical to reflect the varied ways individuals experience diseases based on factors like race, ethnicity, age, and socio-economic status.
Incorporating digital technologies like telemedicine and remote monitoring can alleviate some logistical challenges, but it is essential to ensure individuals receiving medical care are comfortable with these tools. Research has indicated that experiments with patient-focused designs not only have a greater probability of success but also have a tendency to enroll patients more rapidly. Satisfied participants are less likely to withdraw, enhancing data quality and reducing study costs.
Furthermore, it is crucial to acknowledge that the field of clinical experiments is changing, with specific nations demanding domestic clinical research information for official authorization, which can raise the expenses of advancement. Conversely, other nations are introducing innovation pathways to expedite approvals, acknowledging the value of efficient and effective experimentation conduct.
Ultimately, the success of recruiting individuals in Latin America depends on clear communication, inclusivity, and the adoption of modern technologies to minimize the burden on patients, while always prioritizing the patient experience.
The Role of Technology in Streamlining Recruitment
Utilizing technology is vital in improving recruitment strategies for medical studies, especially in Latin America. The integration of electronic health records (EHRs) and patient databases significantly improves the efficiency and accuracy of identifying suitable test participants. Using telemedicine and mobile health applications can also tackle the historical challenges of diversity and inclusion in research.
EHRs, when combined with AI-driven analytics, can pinpoint specific demographic groups, such as women, older adults, children, and Hispanic and Latino communities, streamlining the recruitment process. These technological solutions not only save time but also ensure that diverse populations, which have been underrepresented in trials, are included, leading to more comprehensive and reliable data on treatment efficacy across various groups.
Moreover, telemedicine platforms and mobile apps serve as vital tools for overcoming barriers that have historically limited minority participation in medical research. These include access to healthcare facilities, financial constraints, language differences, and cultural disparities. By enabling remote consent and participation, these technologies can help establish trust with communities that have been hesitant about research due to past injustices like the Tuskegee Syphilis Study.
Furthermore, the utilization of digital engagement tools for individuals, wearable devices, and sensors can improve the participant experience by facilitating quicker outcome evaluations and real-time data transmission. This method not only reduces human mistakes and delays but also enables prompt safety analysis, contributing to a safer and more efficient testing process.
The significance of diversity, equity, and inclusion in experimentation is echoed by experts like Daniel J. Herron, who emphasizes the necessity of involving individuals in planning trials and ensuring that information is accessible and understandable. With technology, it is possible to reach out to populations with varied lived experiences and conditions, which is essential for the integrity and applicability of research findings.
Ensuring Informed Consent and Patient Privacy
The foundation of performing medical experiments is honoring the independence of participants, which is maintained through the informed agreement procedure and the safeguarding of patient confidentiality. In the region of America, ethical and legal compliance is not just a regulatory requirement but also a commitment to the dignity of each participant.
The informed consent process must be more than a mere formality; it should be a comprehensive, understandable dialogue. The documents involved are critical to this process, yet they have seen a marked increase in length and complexity over the years, growing from a few pages to often over twenty, with a mandatory list of items exceeding 270 words. This is not without consequence. Many potential participants find these exhaustive documents daunting, contributing to lower enrollment rates, particularly among minority populations. The challenge is to balance the need for thorough information with the imperative of making it accessible and comprehensible, ensuring that participants are genuinely informed.
To address these concerns, stakeholders in the industry have been called upon to simplify consent documents. This simplification includes reducing the reading level and avoiding overly legalistic language, which can be off-putting and obscure the essential information that participants need to make an informed decision. One approach is to focus on facilitating comprehension, not merely presenting isolated facts, and to prioritize the clarity of the information.
In parallel with informed consent, protecting the privacy of clinical trial participants is equally vital. With the advent of technologies such as Artificial Intelligence, biometric data protection has become increasingly relevant. The region of America, while drawing on a rich tapestry of cultures and political histories, faces regulatory gaps that jeopardize the privacy and security of personal information. Advances in biometric technology underscore the urgency for improved protection measures, like those in the European Union, including encryption and multi-factor authentication.
Anticipating the future, the region of South America is ready to assume a more prominent position on the international platform, with Brazil's forthcoming leadership of the G20 in 2024. This presents a unique opportunity to advance health and health equity in the region, which is crucial given the diverse public health challenges faced. In the midst of this context, the execution of experiments in the area can be a guiding light of advancement, given that moral recruitment practices and individual confidentiality are given priority. With a focus on making the patient experience a top priority, including in the informed consent process, Latin America can enhance its growing medical and scientific expertise while fostering an environment where clinical trials can thrive.
Conclusion
In conclusion, embarking on clinical trials in Latin America requires a comprehensive understanding of the region's unique challenges and opportunities. To ensure successful patient recruitment, it is crucial to address the underrepresentation of minority groups in research, overcome access barriers and cultural nuances, and integrate trials with clinical practice. Building effective advertising strategies, understanding the target audience, utilizing digital recruitment campaigns, and engaging with patient communities and advocacy groups are essential components of enhancing recruitment efforts.
Additionally, ensuring site engagement and support, addressing health equity and diversity, tracking progress and metrics, and leveraging technology are key factors in streamlining patient recruitment strategies. These strategies foster inclusivity, trust, and patient-centricity, while advancing medical science and improving healthcare outcomes in Latin America.
Moreover, prioritizing informed consent and patient privacy is crucial in upholding ethical research practices. By adopting these approaches, clinical trials in Latin America can not only adhere to scientific standards but also honor the collaborative spirit that drives progress in medical research.
Ultimately, by embracing accurate and detailed information in a formal and professional manner, clinical trials in Latin America can optimize patient recruitment strategies, foster inclusivity, and advance healthcare outcomes for diverse populations in the region.
Learn how bioaccess™ can help you optimize your patient recruitment strategies in Latin America.




