Overview
Patient recruitment strategies in Chile are pivotal for the success of clinical trials, emphasizing the importance of understanding the regulatory framework, engaging local healthcare providers, and utilizing culturally relevant outreach methods. Effective recruitment hinges on strict compliance with regulations, fostering strong relationships with regulatory bodies, and employing tailored approaches that resonate with the community. This strategy not only enhances participant engagement but also improves retention in clinical studies, ultimately contributing to more successful outcomes in clinical research.
Introduction
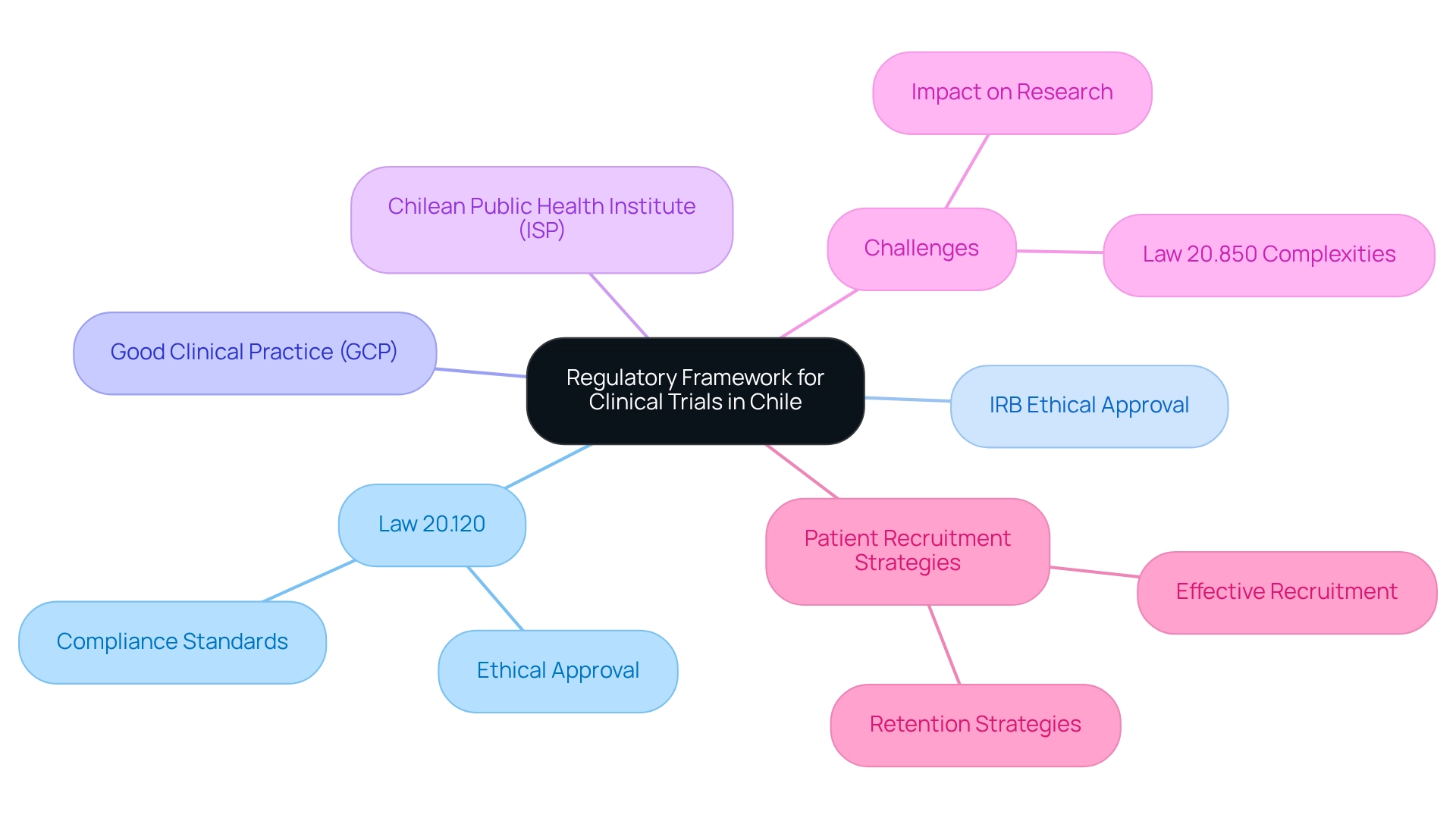
Navigating the intricate landscape of clinical trials in Chile demands a comprehensive understanding of the regulatory framework that governs them. With Law 20.120 at the forefront, researchers are required to adhere to stringent ethical guidelines and secure necessary approvals from Institutional Review Boards (IRBs) to ensure compliance and uphold public trust.
The Chilean Public Health Institute (ISP) plays a pivotal role in this process, overseeing trials to ensure alignment with both national and international standards. As the research environment evolves, however, challenges such as bureaucratic delays and complex regulations emerge as significant hurdles.
This article explores the essential components of conducting clinical trials in Chile, from grasping the regulatory bodies involved to implementing effective patient recruitment strategies tailored to the unique needs of the local population. By examining these dimensions, stakeholders can better navigate the complexities of clinical research, ultimately advancing medical innovation in the region.
Understand the Regulatory Framework for Clinical Trials in Chile
Chile's regulatory structure for medical studies is primarily governed by Law 20.120, which establishes comprehensive criteria for conducting research involving human subjects. This law mandates the acquisition of ethical approval from Institutional Review Boards (IRBs) and adherence to Good Clinical Practice (GCP) guidelines, both critical for ensuring ethical and legal compliance in studies. Such adherence not only cultivates trust among potential participants but also reassures stakeholders about the integrity of the research process.
The Chilean Public Health Institute (ISP) plays a pivotal role in this ecosystem, overseeing trials to ensure they meet both national and international standards. With a workforce exceeding 9 million and a GDP surpassing $277 billion, Chile presents a robust environment for medical research, particularly through effective patient recruitment strategies and retention.
bioaccess® is dedicated to ensuring information security and client trust through its comprehensive grievance and data protection procedures, addressing any concerns in compliance with applicable laws. However, challenges persist. Recent concerns have been raised regarding the complexities of Law 20.850, which some researchers believe could hinder regional scientific and entrepreneurial efforts, potentially affecting patient recruitment strategies in Chile.
Thus, understanding and navigating these regulations is vital for effective patient recruitment and retention in research studies throughout the region. Furthermore, insights from case studies on governance capacity and local innovation underscore the necessity for long-term capacity-building initiatives to enhance local research capabilities. Emphasizing fair data exchange and skill development can also benefit all participants, ultimately leading to more effective research studies.
Identify Key Regulatory Bodies and Their Roles
In Chile, the regulatory framework governing research studies is primarily overseen by the Ministry of Health (MoH) and the Public Health Institute (ISP). The MoH formulates vital health policies and regulations, while the ISP is tasked with endorsing clinical studies and ensuring compliance with health standards. Furthermore, local ethics committees play an essential role in reviewing research protocols, safeguarding participant welfare, and upholding ethical practices. Engaging these governing entities early in the planning stage is crucial; it streamlines the approval process and significantly enhances the likelihood of successful patient recruitment strategies in Chile.
For instance, successful partnerships with the ISP have demonstrated that implementing patient recruitment strategies can lead to more efficient study arrangements and increased participant involvement. Additionally, initiatives like the collaboration between bioaccess™ and Caribbean Health Group in Colombia illustrate how strategic alliances can position regions as prominent sites for research trials, potentially offering valuable insights for Chile. As highlighted in the case study 'Cultural Sensitivity in Informed Consent,' cultural variations across Latin America can impact informed consent processes, as patients often accept physician recommendations without question, which may pose ethical challenges in research studies. Thus, harmonizing local cultural practices with ethical guidelines is imperative to protect patient rights while respecting local customs in research.
Moreover, the Chilean Ministry of Health and the Public Health Institute of Chile (ISP) oversee the regulation and monitoring of stem cell therapies within the country, which hold the promise to revolutionize healthcare by providing hope for diseases previously considered untreatable. As of 2025, the MoH is actively refining its processes, striving to cultivate a more responsive environment for health research—an essential step for advancing innovative medical technologies in the region.
Overcome Challenges in Achieving Regulatory Compliance
Achieving compliance with regulations in Chile presents significant challenges, primarily due to bureaucratic delays and the intricate nature of the approval process. In 2025, these obstacles remain a critical issue, with numerous studies experiencing prolonged timelines for approval. Notably, 52 percent of worldwide clinical studies occur beyond the U.S., underscoring the necessity of efficiently managing regional compliance frameworks.
To effectively navigate these complexities, it is essential to establish strong relationships with regulatory bodies and community ethics committees. This proactive involvement fosters teamwork and can lead to more effective communication regarding testing requirements. Furthermore, preparing thorough documentation that complies with regional regulations is vital for accelerating the approval process. This includes ensuring that all experimental protocols are meticulously designed to meet the unique requirements of Chile's compliance framework. Leveraging local expertise, such as hiring regulatory consultants with a profound understanding of the Chilean landscape, can significantly enhance the likelihood of successfully navigating these regulatory challenges.
A notable case study illustrates the importance of strategic planning in overcoming these obstacles. In Chile, researchers faced setbacks due to engaged civil society and regular protests impacting research operations. By proactively engaging with stakeholders and adapting their strategies to the socio-political context, they ensured that their evaluations proceeded smoothly. This example emphasizes the necessity of a well-informed compliance strategy, which not only promotes ethical standards but also bolsters public confidence in research.
As Julio G. Martinez-Clark, CEO, remarked about Colombia, "Colombia appears to be the sole nation in Latin America vigorously advertising itself as a leading research destination in the area." This highlights the need for Chile to similarly enhance its oversight procedures to attract more research studies and stimulate economic growth.
To support these efforts, bioaccess® offers comprehensive research study management services, including:
- Feasibility assessments
- Site selection
- Compliance reviews
- Study setup
- Import permits
- Project management
- Reporting
By positioning itself as a prominent Contract Research Organization, bioaccess® is well-equipped to assist in navigating regulatory challenges in Chile and improving the success of research studies.
Implement Effective Patient Recruitment Strategies
A profound comprehension of the regional population and their healthcare needs is essential to effectively execute patient recruitment strategies in Chile. Partnering with local healthcare providers significantly enhances awareness of clinical studies, as these professionals frequently serve as reliable sources of information for prospective participants. Leveraging social media platforms and community outreach initiatives can further engage individuals, informing them of available studies. It is crucial that recruitment materials are culturally aware and accessible in Spanish to connect with the intended audience.
Utilizing patient advocacy organizations can also have a critical impact on engaging underrepresented communities, thereby enhancing diversity in study involvement. For instance, a pilot study on an electronic patient-reported outcome (ePRO) intervention for breast cancer patients in Mexico achieved an impressive 100% retention rate, showcasing the effectiveness of tailored approaches in boosting participant engagement. Moreover, eight systematic reviews have evaluated interventions for caregivers, highlighting effective strategies that can be adapted for patient recruitment. Consistently evaluating and modifying recruitment approaches based on participant input can lead to improved enrollment rates, ensuring that studies reflect the varied needs of the community. Furthermore, traits indicating strong support for research and rural methods have been observed to be more effective in attracting participants, suggesting that focused strategies in these areas may yield better outcomes.
By concentrating on patient recruitment strategies in Chile, studies can achieve greater success and contribute to the advancement of medical technology. bioaccess® offers extensive management services for studies, including pilot studies, first-in-human studies, early-feasibility studies, pivotal studies, and post-market follow-up studies. This reinforces its commitment to facilitating effective patient recruitment in the Medtech sector, bolstered by collaborations such as that with Caribbean Health Group, which aims to position Barranquilla as a leading destination for clinical trials in Latin America.
Conclusion
Navigating the landscape of clinical trials in Chile necessitates a comprehensive understanding of the regulatory framework established by Law 20.120, alongside the pivotal roles of key regulatory bodies such as the Ministry of Health and the Public Health Institute. These entities are indispensable in ensuring compliance with ethical standards and Good Clinical Practice, fostering public trust and supporting the integrity of research. Despite the promising environment for clinical trials, challenges such as bureaucratic delays and regulatory complexities present significant hurdles that stakeholders must confront.
Effective patient recruitment strategies stand as a cornerstone for the success of clinical trials. By engaging with local healthcare providers, employing culturally sensitive outreach, and leveraging patient advocacy groups, researchers can significantly enhance awareness and participation in trials. Tailoring recruitment efforts to address the diverse needs of the local population not only boosts enrollment rates but also enriches the quality of clinical research conducted in the region.
In summary, a thorough understanding of the regulatory framework, proactive engagement with local stakeholders, and the implementation of effective recruitment strategies are essential for propelling clinical research in Chile. By overcoming existing challenges and fostering collaboration, the potential for medical innovation and improved healthcare outcomes can be realized, ultimately benefiting both researchers and the communities they serve.
Frequently Asked Questions
What is the primary law governing medical studies in Chile?
The primary law governing medical studies in Chile is Law 20.120, which establishes comprehensive criteria for conducting research involving human subjects.
What are the requirements for conducting medical research in Chile?
Researchers must obtain ethical approval from Institutional Review Boards (IRBs) and adhere to Good Clinical Practice (GCP) guidelines to ensure ethical and legal compliance in studies.
What role does the Chilean Public Health Institute (ISP) play in medical research?
The Chilean Public Health Institute (ISP) oversees trials to ensure they meet both national and international standards.
How does Chile's economic environment support medical research?
Chile has a workforce exceeding 9 million and a GDP surpassing $277 billion, creating a robust environment for medical research, particularly through effective patient recruitment strategies and retention.
What measures does bioaccess® take to ensure information security and client trust?
bioaccess® ensures information security and client trust through comprehensive grievance and data protection procedures, addressing concerns in compliance with applicable laws.
What challenges are associated with Law 20.850 in Chile?
Recent concerns have been raised about the complexities of Law 20.850, which some researchers believe could hinder regional scientific and entrepreneurial efforts, potentially affecting patient recruitment strategies.
Why is it important to understand and navigate regulations in Chilean medical research?
Understanding and navigating these regulations is vital for effective patient recruitment and retention in research studies throughout the region.
What initiatives are suggested to enhance local research capabilities in Chile?
Long-term capacity-building initiatives, emphasizing fair data exchange and skill development, are suggested to enhance local research capabilities and benefit all participants in research studies.




