Introduction
The FDA's Q-Submission Program is a crucial platform that fosters communication between the agency and stakeholders in the medical device industry. This program allows sponsors, applicants, and other entities to engage with the FDA before formal application submissions, ensuring regulatory expectations are met. By defining the Questions of Interest and the Context of Use for computational models, stakeholders can address regulatory questions effectively.
The program not only enhances the predictability and efficiency of the review process but also promotes transparency and collaboration within the industry. To facilitate understanding and engagement, the FDA has released a draft guidance document on the Q-Submission Program, inviting stakeholders to review and provide comments. This article explores the benefits of using the program, the different types of Q-Submissions, the process overview, key elements of a request, timeline for feedback, integration of Q-Submissions with eQMS, common challenges, and best practices in implementation.
By adhering to these guidelines, stakeholders can navigate the regulatory landscape with confidence and contribute to the development of robust compliance mechanisms.
What is the FDA Q-Submission Program?
The FDA's Q-Submission Program facilitates a vital dialogue between the agency and entities such as sponsors, applicants, and other interested parties prior to formal application presentations. This pre-submission interaction is crucial for clarifying regulatory expectations and ensuring that the development process aligns with the FDA's compliance requirements. In particular, the program allows individuals involved to specify the Questions of Interest and the Context of Use for their computational models, ensuring that the model outputs are properly utilized to address regulatory questions. Using this strategic approach, the FDA aims to reduce risks connected with the use of computational models in regulatory filings, improving the predictability and efficiency of the review process. In accordance with the organization's dedication to transparency and involvement of interested parties, a preliminary guidance document entitled 'Requests for Feedback and Meetings for Medical Device Submissions: The Program for Submissions' has been released. This document provides comprehensive insights into the process of submitting, including mechanisms to obtain feedback and the categorization of various types of document. Stakeholders are encouraged to review and provide comments on the draft guidance to contribute to the development of the final document, ensuring it addresses the community's needs effectively. When interacting with the FDA, submitters are reminded to consider the public nature of their comments and to carefully handle confidential information. The Program showcases the FDA's commitment to protecting public health through strong regulatory mechanisms while promoting innovation and collaboration within the medical device industry.
Benefits of Using the Q-Submission Program
The FDA program is crucial in facilitating successful regulatory filings for medical devices. By engaging with the FDA early through this program, sponsors can gain invaluable feedback that informs product development and ensures adherence to regulatory standards. This proactive approach is crucial, as the information for regulatory filings is multifaceted, encompassing experiment reports, meeting notes, and more, which can span thousands of pages for a single filing. The submission program not only helps in proactively identifying possible problems but also improves openness and promotes a cooperative atmosphere between the FDA and interested parties. Such collaboration is vital to achieving a balance between safety and speed, as highlighted by the paper "Harmonizing Safety and Speed: A Human-Algorithm Approach to Enhance the FDA's Medical Device Clearance Policy". By following this program, individuals involved can effectively navigate the intricacies of regulatory filings, ultimately promoting public health by guaranteeing the safety, efficacy, and security of medical devices.
Types of Q-Submissions
The FDA's Q-Submission Program is a complex system created to facilitate a variety of requests, each customized to the specific requirements of individuals navigating the regulatory landscape. Here are key types of submissions:
-
Pre-Submission (Pre-Sub): This type is vital for stakeholders seeking FDA's input on specific questions or concerns associated with their medical product or study. It proves invaluable for clarifying regulatory requirements and obtaining guidance on pivotal issues such as study design and the scientific or technical facets of a document.
-
Investigational New Drug (IND) Meeting Requests: These are critical for sponsors or applicants poised to commence clinical trials. 'IND applications open the door for discussions with the FDA regarding study design, data requisites, or other pertinent topics, ensuring that the proposed clinical research meets the regulatory standards.'.
-
For high-risk medical devices requiring FDA approval, PMA applications are the gateway. Interacting with the FDA in the early stages through the Q-Submission Program can assist in identifying and resolving potential issues, improving the completeness and quality of the application.
-
These applications are vital for sponsors or applicants seeking FDA's approval for biological products. These terms 'Submission Program'.
-
New Drug Application (NDA) Submissions: Applicants or sponsors aiming for FDA approval of new drugs embark on NDA submissions. These words Submission are still exist. Fix this error and return result.
The Program is not just a procedural pathway but a strategic framework designed to ensure that stakeholders can effectively leverage computational models to address regulatory questions. It involves defining the Questions of Interest and the Context of Use (COU), ensuring that the model's outputs are optimally utilized to inform regulatory filings. The FDA encourages public feedback on these processes, with the most recent draft guidance on the Q-Submission Program providing enhanced clarity on different types of submissions and feedback mechanisms, and inviting comments until May 14, 2024.
To ensure safety, effectiveness, and security are prioritized by the FDA for a wide range of products, it is crucial for individuals involved to have a comprehensive understanding of the scope and requirements of the submission process. The most recent FDA announcements and publications, including those on medical device filings and advertising regulations for prescription drugs, demonstrate the Agency's dedication to transparency and public involvement in the regulatory process. Stakeholders are encouraged to submit comments carefully, avoiding confidential information, to partake in shaping the regulatory environment.
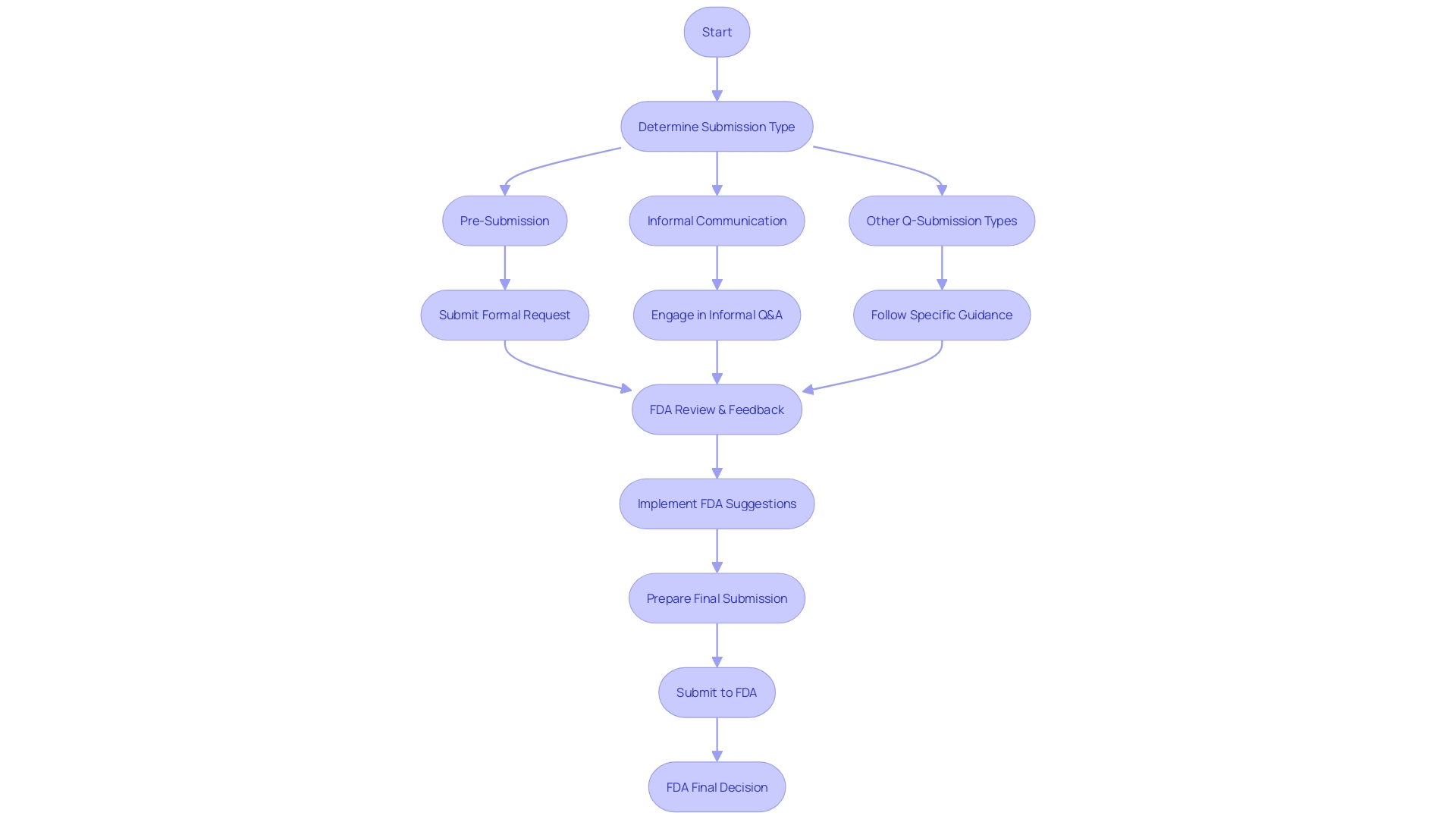
Pre-Submission (Pre-Sub) Process Overview
The FDA's Q-Submission Program, including the Pre-Submission (Pre-Sub) process, plays a crucial role in ensuring effective communication between interested parties and the regulatory body prior to the formal submission of an application or report. This process encompasses a series of steps designed to clarify and resolve potential questions or issues early on. At first, interested parties draft a Pre-Sub request outlining the subjects for discussion, along with pertinent questions and any supporting documents or data. This request is then electronically submitted to the FDA. Upon receiving it, the FDA evaluates the request, which may include seeking additional information or clarification from the interested parties. If considered essential, a Pre-Sub meeting is organized, providing individuals with an opportunity to present their concerns and inquiries, and to obtain the FDA's feedback and guidance. The final outcome of this process is the FDA's written response, which offers interested parties with the agency's perspectives and suggestions, thus enabling a more thorough, precise, and compliant submission.
The FDA's draft guidance titled 'Requests for Feedback and Meetings for Medical Device Submissions: The Program' explains the different methods in which individuals can interact with the FDA. This document, set to supersede the previous guidance issued on June 2, 2023, aims to clarify the scope of Q-Sub types and the methods for obtaining feedback. Stakeholders are encouraged to submit comments on the draft guidance by May 14, 2024, to influence its finalization. These initial engagements with the FDA are crucial for all parties involved, especially when contemplating investigational device exemptions (IDEs) for devices that have not yet been approved for marketing, to collect the required safety and effectiveness data.
In the broader context of regulatory oversight, the FDA ensures public health protection by regulating the safety, efficacy, and security of various products, including medical devices. The agency's recent publication, 'Direct-to-Consumer Prescription Drug Advertisements: Presentation of the Major Statement in a Clear, Conspicuous, and Neutral Manner,' exemplifies its commitment to clear communication standards. Public comments are valued by the agency and contribute to the development of guidelines that reflect the latest regulatory requirements and best practices.
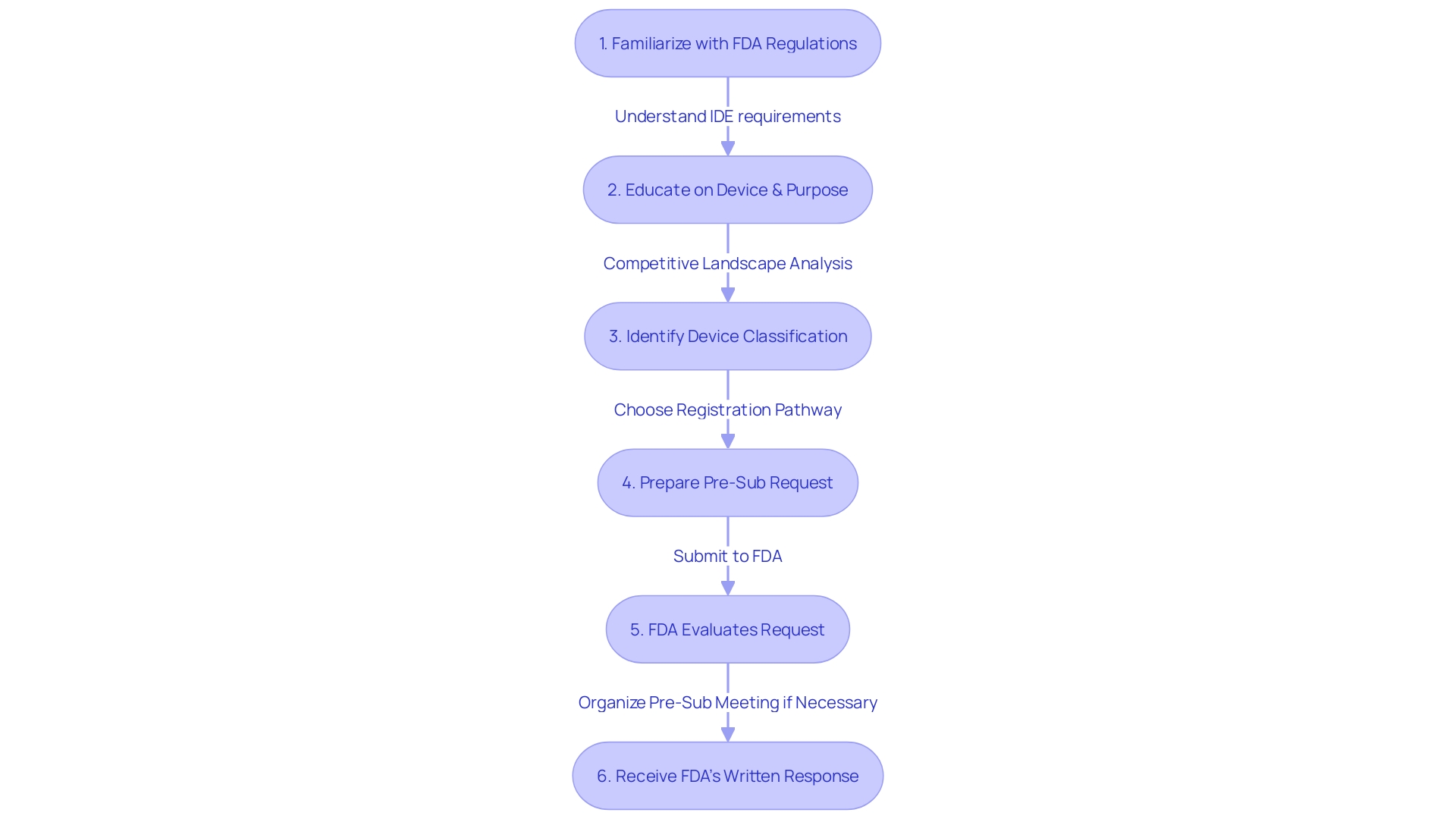
Key Elements of a Q-Submission Request
Developing a successful Q-Submission inquiry is a crucial stage in interacting with the FDA for medical device applications. To enhance the probability of acquiring valuable feedback, individuals involved must make certain that their entry is transparent, succinct, and comprehensive. A well-prepared request should incorporate the following elements:
-
Background Information: A succinct overview of the product, study, or issue is essential. This foundational knowledge equips the FDA with the necessary context to evaluate the document.
-
Specific Questions: Articulate the particular questions or issues that require the FDA's feedback. Precision in these queries is vital for a focused and productive exchange.
-
Supporting Documentation: Present relevant evidence such as study protocols, scientific literature, or prior FDA correspondences. This body of work aids the FDA's understanding and review process.
-
Defining the aims and anticipated outcomes of the submission provides the FDA with a clear vision of what the stakeholder intends to achieve.
-
Proposed Approach: Outlining a proposed solution or methodology for the issues at hand can jumpstart the dialogue and guide the FDA's recommendations.
Incorporating these elements with careful attention to detail and consistency across all documentation, such as clinical evaluation plans, clinical evaluation reports, and risk management files, is crucial to avoid disconnects between data and conclusions. Moreover, utilizing post-market surveillance data with equal rigor as primary data can greatly enhance the application.
The FDA's draft guidance on 'Requests for Feedback and Meetings for Medical Device Submissions: The Program' offers additional clarity on the types of submissions and the methods for obtaining feedback. Stakeholders are encouraged to review and comment on this draft guidance to contribute to its refinement before finalization.
Additionally, the FDA emphasizes the importance of confidentiality in public comments. It is the submitter's responsibility to exclude sensitive information from their comments if they do not wish it to be public. If confidential information needs to be shared, it should be done through a written document as per the provided instructions.
By conducting focus groups and interviews, the FDA collects valuable insights into consumer attitudes and beliefs, which highlights the significance of comprehending the target audience's perspectives when formulating a submission request. It is essential to communicate in a manner that resonates with reviewers, using consumer-friendly language and presenting information in a clear and accessible way.
By following these principles and using the FDA's feedback channels, interested parties can successfully navigate the regulatory environment and progress their medical device applications.
How to Prepare a Q-Submission Meeting Request
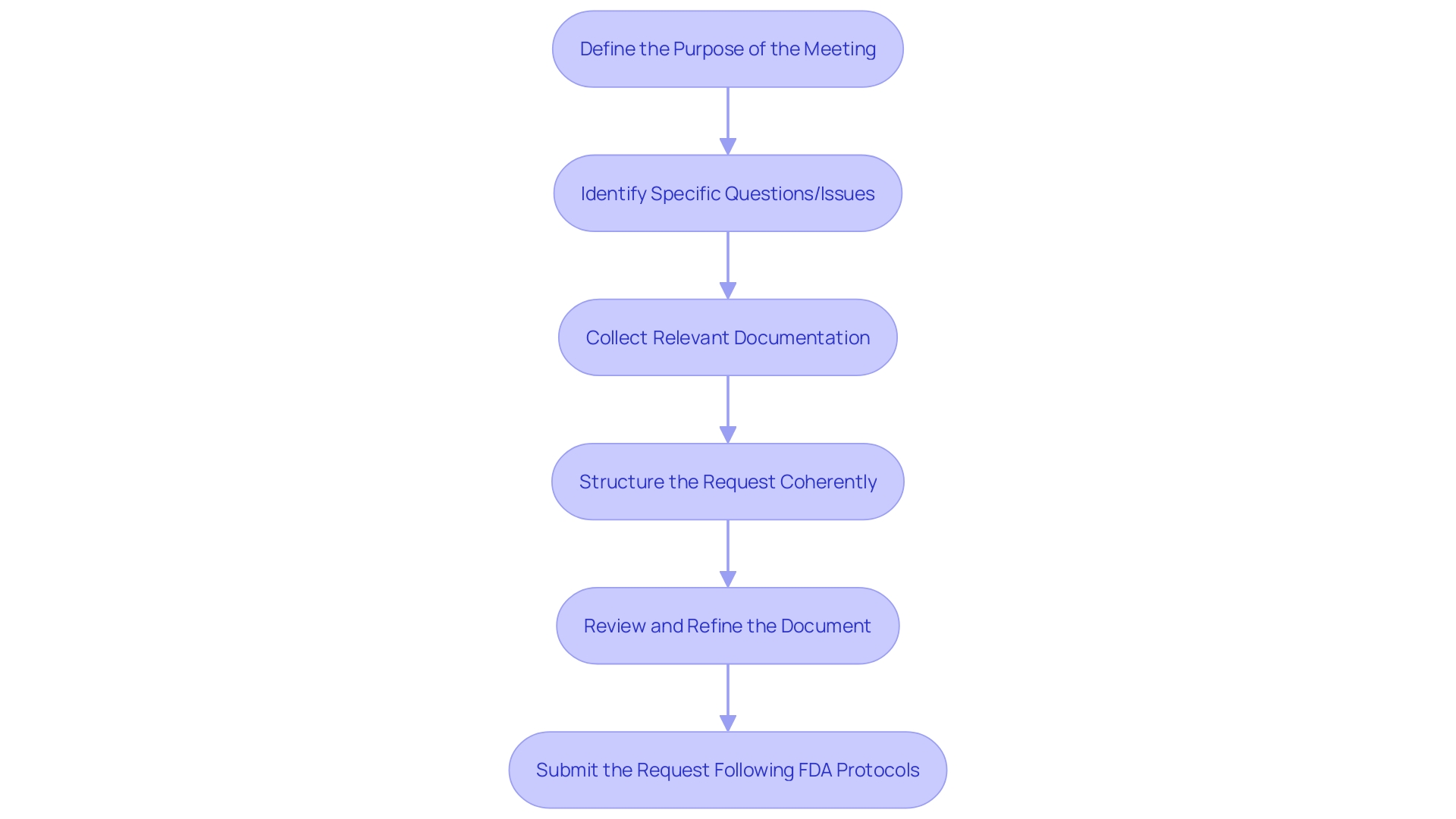
To prepare a Q-Submission meeting request for the FDA, stakeholders are advised to undertake the following meticulously structured steps:
-
Clearly Define the Purpose: Begin with a clear understanding of the meeting's objective, which could range from soliciting feedback on a particular issue, discussing study designs, to addressing regulatory requirements. A well-defined purpose is crucial for an organized meeting request.
-
Identify Specific Questions or Issues: List the precise questions or issues to be addressed. These should be articulated clearly and succinctly to ensure a focused and effective discussion.
-
Collect Relevant Documentation: Assemble supporting materials such as study protocols, scientific literature, or past communications with the FDA. These documents are vital in providing context to the issues discussed and support your positions.
-
Structure the Request Coherently: Organize your entry starting with a concise introduction and background, followed by the identified questions or issues, the supporting documentation, and the proposed approach or solution.
-
Review and Refine the Document: Conduct a thorough review of the meeting request to confirm its clarity, accuracy, and completeness. Eliminate any extraneous information to maintain a concise and targeted request.
-
Submit the Request: Utilize the proper electronic submission portal or email address, following FDA's protocols, to submit your meeting request.
By adhering to these steps, stakeholders can effectively communicate their goals to the FDA and facilitate a constructive dialogue. This method is in accordance with the FDA's draft guidance on the 'Requests for Feedback and Meetings for Medical Device Submissions: The Program for Submitting Questions', which, when completed, will provide more clarity on types of submissions and mechanisms for feedback. Stakeholders are encouraged to review this guidance and provide comments before the finalization date.
Timeline for Q-Submission Feedback
Managing the process of ensuring FDA compliance follows a set of expected stages, each with its own usual timeframe, although the precise duration can change depending on the specifics of the filing and the current workload of the FDA. At first, interested parties will receive a confirmation from the FDA soon after the Q-Submission, verifying that the agency has received the document. This acknowledgment is typically issued within days.
Following the acknowledgment, the FDA embarks on a thorough review and evaluation phase. This critical step involves a detailed examination of the questions or issues raised, with the complexity of the submission and resource availability influencing the time required, potentially spanning from several weeks to a few months.
If the situation requires a meeting, the FDA collaborates with interested parties to establish a date, which may take extra weeks or months to finalize depending on mutual availability. The following Pre-Sub meeting provides an opportunity for interested parties to discuss their queries directly with the FDA, gaining valuable feedback and guidance.
After the meeting, interested parties can expect a formal written response from the FDA. This post-meeting feedback, encompassing answers to raised questions and further recommendations, might take several weeks to a couple of months to be communicated.
Throughout this process, it's crucial for individuals involved to consider these timeframes when planning their submissions. By submitting early and maintaining proactive communication with the FDA, individuals involved can facilitate a smoother and more effective feedback process.
Moreover, the FDA continuously strives to enhance the Q-Submission program, as evidenced by the agency's recent announcement of the draft guidance entitled 'Requests for Feedback and Meetings for Medical Device Submissions: The Q-Submission Program.' This new guidance, set to replace the prior edition starting in June 2023, aims to clarify and expand upon the varieties of Q-Submissions, delineate feedback mechanisms, and present improved examples, highlighting the FDA's dedication to transparency and involvement of interested parties.
Stakeholders can submit comments on this draft guidance by the specified deadline to contribute to the development of the final guidance. Such participatory measures reinforce the FDA's dedication to safeguarding public health while fostering innovation and regulatory quality through standards that prioritize transparency, stakeholder involvement, and due process.
Electronic Quality Management Systems (eQMS) for FDA Compliance
The integration of Q-Submissions with Electronic Quality Management Systems (eQMS) is instrumental in bolstering an organization's compliance management framework. This strategic approach facilitates a cohesive flow of information and data between the submission process and eQMS. It minimizes manual labor, enhances data integrity, and reinforces compliance oversight.
-
Centralized Data Management: By harmonizing Q-Submissions with eQMS, there is a unified platform for managing regulatory interactions and associated documentation. This integration ensures all pertinent details are readily available, systematically organized, and easily retrievable for audits or regulatory scrutiny.
-
Workflow Automation: Automating workflow connected to Q-Submissions and compliance management is another benefit of this integration. It simplifies the generation, assessment, endorsement, and tracking of request documents, as well as the management of feedback and subsequent steps.
-
Data Traceability and Auditability: With the integrated system, all regulatory compliance-related interactions and activities are meticulously recorded and auditable. This level of traceability promotes transparency, accountability, and evidential compliance with regulatory demands.
-
Reporting and Analytics: The integrated system also supports the generation of detailed reports and analytics on Q-Submissions, compliance tasks, and associated metrics. This capability assists organizations in tracking their performance, discerning trends, and making informed decisions to refine their compliance management operations.
-
Collaboration and Communication: The synergy between Q-Submissions and eQMS enhances collaboration and communication among all stakeholders in the compliance management chain. It allows for the instantaneous exchange of information, feedback, and updates, thus improving overall efficacy and ensuring that each participant is informed and in alignment.
Utilizing eQMS capabilities and integrating them with the submission process enables organizations to enhance their compliance management practices, comply with regulatory requirements, and ultimately enhance the quality and safety of their products or services. As the FDA announces draft guidance on the Q-Submission Program, offering clarity on Q-Sub types and feedback mechanisms, the importance of such integrations becomes increasingly clear. This guidance is set to replace the previous document issued on June 2, 2023, highlighting the evolving nature of regulatory frameworks and the need for adaptive compliance management systems.
Common Challenges and Best Practices in Q-Submission and eQMS Implementation
The landscape of regulatory compliance and quality management in industries like medical device manufacturing, pharmaceuticals, and life sciences is rapidly advancing. As organizations navigate the implementation of Q-Submissions and Electronic Quality Management Systems (eQMS), they encounter a variety of challenges that can be systematically addressed through proven best practices.
Regulatory Complexity: Understanding and adhering to the intricate web of regulations is paramount. Organizations can engage with ongoing education and collaboration with industry experts to remain abreast of changes and facilitate compliance during Q-Submissions and eQMS implementation.
Resource Allocation: Dedicated resources are crucial for the successful adoption of these systems. A strategy that encompasses training, role definition, and potentially, external partnerships can provide the necessary support for these critical initiatives.
Change Management: Integrating new systems can disrupt existing operations. A robust change management strategy, complete with clear communication and training, can ease the transition and foster an environment that values compliance.
Data Security and Privacy: With sensitive information at stake, protecting data through encryption, access controls, and backups is essential to prevent unauthorized access and maintain trust.
Continuous Improvement: Q-Submissions and eQMS should be dynamic processes that evolve with the industry. Regular audits, performance monitoring, and feedback mechanisms can drive improvement and ensure responsiveness to new regulatory demands.
By embracing these best practices, organizations can enhance their compliance management processes, thereby improving the quality and safety of their offerings while positioning themselves for success in a competitive and regulated market.
Conclusion
In conclusion, the FDA's Q-Submission Program is a crucial platform that fosters communication and collaboration between the agency and stakeholders in the medical device industry. By engaging with the FDA early through this program, sponsors and applicants can gain valuable feedback that informs product development and ensures adherence to regulatory standards.
Creating an effective Q-Submission request is crucial for obtaining valuable feedback from the FDA. Stakeholders should ensure their submission is clear, concise, and thorough, incorporating elements such as background information, specific questions, supporting documentation, objectives and goals, and a proposed approach.
Integrating Q-Submissions with Electronic Quality Management Systems (eQMS) offers benefits such as centralized data management, workflow automation, data traceability and auditability, reporting and analytics, and enhanced collaboration and communication.
While implementing Q-Submissions and eQMS, organizations may encounter challenges such as regulatory complexity, resource allocation, change management, and data security and privacy. These challenges can be addressed through best practices such as ongoing education and collaboration, dedicated resources, change management strategies, data protection measures, and a focus on continuous improvement.
By adhering to these guidelines and utilizing the FDA's feedback mechanisms, stakeholders can effectively navigate the regulatory landscape, contribute to the development of robust compliance mechanisms, and ensure the safety, effectiveness, and security of medical devices. The FDA's commitment to transparency and stakeholder engagement is evident through the release of draft guidance documents, inviting stakeholders to review and provide comments, thus shaping the regulatory environment to meet the community's needs effectively.




