Introduction
Quality management in medical devices represents a comprehensive and systematic approach crucial for ensuring that products meet rigorous safety and efficacy standards throughout their lifecycle. This intricate process involves meticulous planning, control, and enhancement of all elements that influence product quality. Effective quality management systems (QMS) are indispensable, encompassing a broad spectrum of activities from design and development to production and post-market surveillance, all while adhering to stringent regulatory requirements and industry best practices.
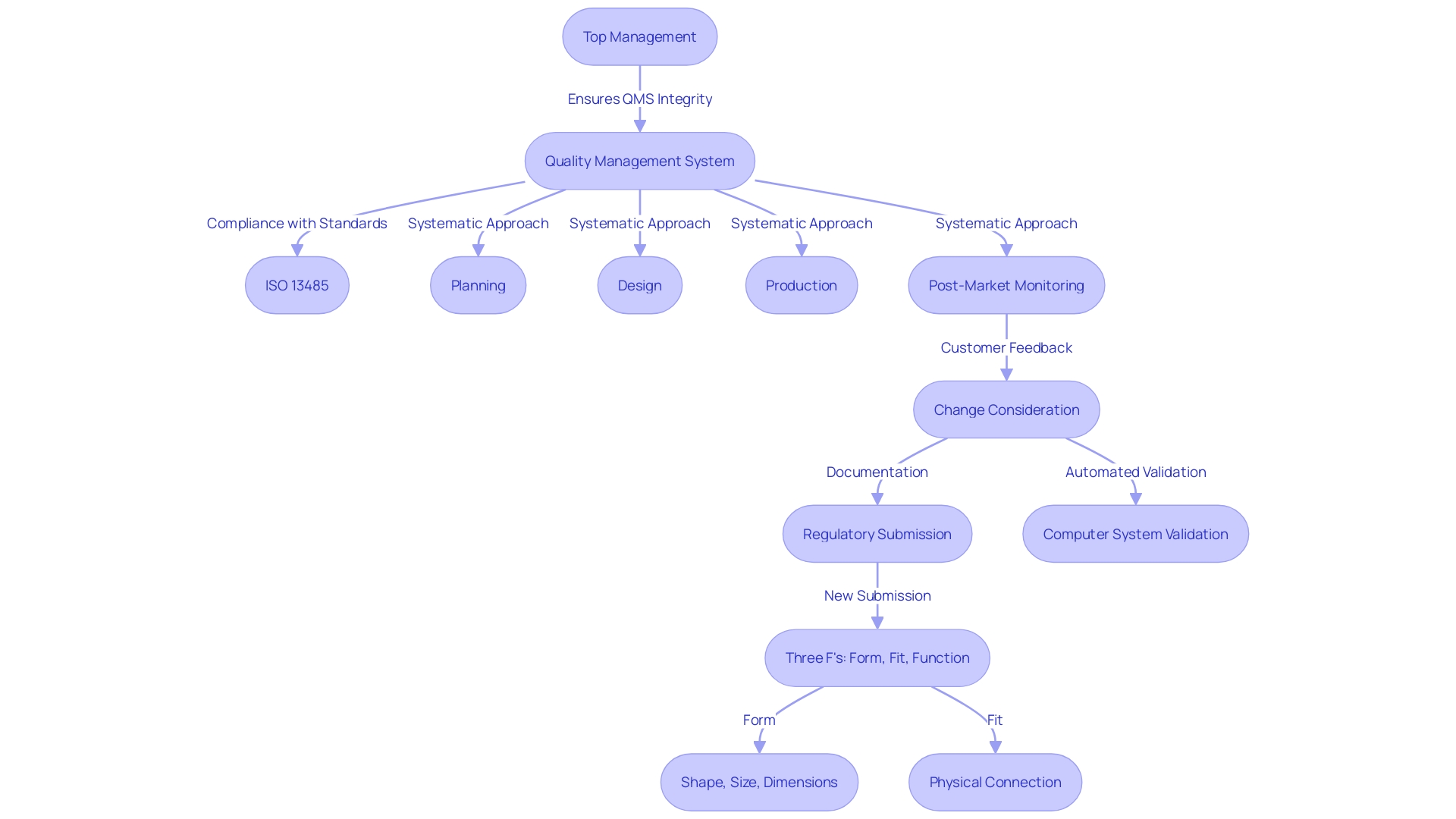
Central to quality management in medical device manufacturing is ISO 13485, which mandates organizations to establish and maintain documented procedures for managing all QMS-related documents. This includes ensuring documents are approved, reviewed, updated, and accessible to prevent the unintended use of obsolete documents. Top management plays a pivotal role in committing to meeting customer requirements and maintaining the QMS's integrity during changes.
Adequate resources must be allocated towards human resources, infrastructure, and work environments to support the QMS effectively.
The dynamic nature of the regulatory landscape, driven by evolving standards and innovations such as Software as a Medical Device (SaMD), presents ongoing challenges and opportunities for improvement. Embracing automation in compliance processes, as encouraged by regulatory bodies like the FDA, is increasingly critical to enhancing efficiency and maintaining high standards of quality and patient safety. This article delves into the multifaceted components of quality management systems, the regulatory framework governing medical devices, and the pivotal role of quality engineers in ensuring safety and efficacy across the product lifecycle.
Understanding Quality Management in Medical Devices
Quality management in medical devices is an intricate and systematic approach dedicated to ensuring that products consistently meet stringent safety and efficacy standards throughout their lifecycle. This comprehensive process encompasses planning, controlling, and improving all aspects that influence product excellence. Efficient management systems (QMS) are crucial and encompass a broad range of activities from design and development to production and post-market monitoring, ensuring compliance with legal standards and industry best practices.
'ISO 13485 is the foundation for management standards in healthcare product manufacturing, requiring that organizations create and uphold recorded processes for handling all documentation associated with the QMS requirements.'. This includes the approval, review, updating, and retrieval of documents to ensure accessibility and prevent the unintended use of obsolete documents. Top management must demonstrate their commitment by ensuring customer requirements are met and maintaining the QMS' integrity during changes. Organizations are also required to allocate adequate resources towards human resources, infrastructure, and work environments to support their QMS.
Jennifer Mascioli-Tudor's insights highlight the necessity of agility and adaptability in building a robust QMS. A skilled quality and compliance expert must have outstanding project management abilities, as the range of QMS goes beyond design and the product itself to include all related aspects that contribute to the final healthcare item.
Additionally, the changing environment of compliance requirements greatly influences the creation and market introduction of medical equipment. Regulatory bodies such as the FDA encourage a shift from manual validation processes to more automated approaches using computer systems. 'This transition aims to enhance efficiency and compliance, as emphasized by industry experts and reflected in recent oversight initiatives.'.
The significance of effective management in guaranteeing that each apparatus meets set standards is highlighted by compliance frameworks such as the GMP, which offer a systematic method for upholding consistent excellence and conformity to international guidelines. 'The complexity of these processes, combined with compliance constraints and confidentiality concerns, presents challenges that organizations must navigate to maintain efficiency.'.
In conclusion, quality management in healthcare instruments is a multifaceted process that demands a strategic and well-resourced approach to ensure compliance, safety, and efficacy. Embracing best practices, staying agile, and continuously improving the QMS are crucial for success in this highly regulated industry.
Regulatory Framework for Medical Devices
The regulatory environment for medical products is intricate and region-specific, influencing every phase from development to market entry. In the United States, the Food and Drug Administration (FDA) mandates compliance with standards such as ISO 13485 and oversees the classification process of products. Devices must undergo appropriate pathways like Premarket Notification (510(k)), Premarket Approval (PMA), or De Novo process before they can be legally marketed. Each classification level carries different patient risk values, making accurate categorization crucial.
In Europe, the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR) impose rigorous requirements for the safety and performance of instruments. These regulations necessitate enhanced risk management practices throughout the lifecycle of a product, from design to post-market surveillance. By integrating risk management principles, manufacturers can proactively address potential risks, ensuring the development of high-quality devices that comply with standards and optimize patient outcomes.
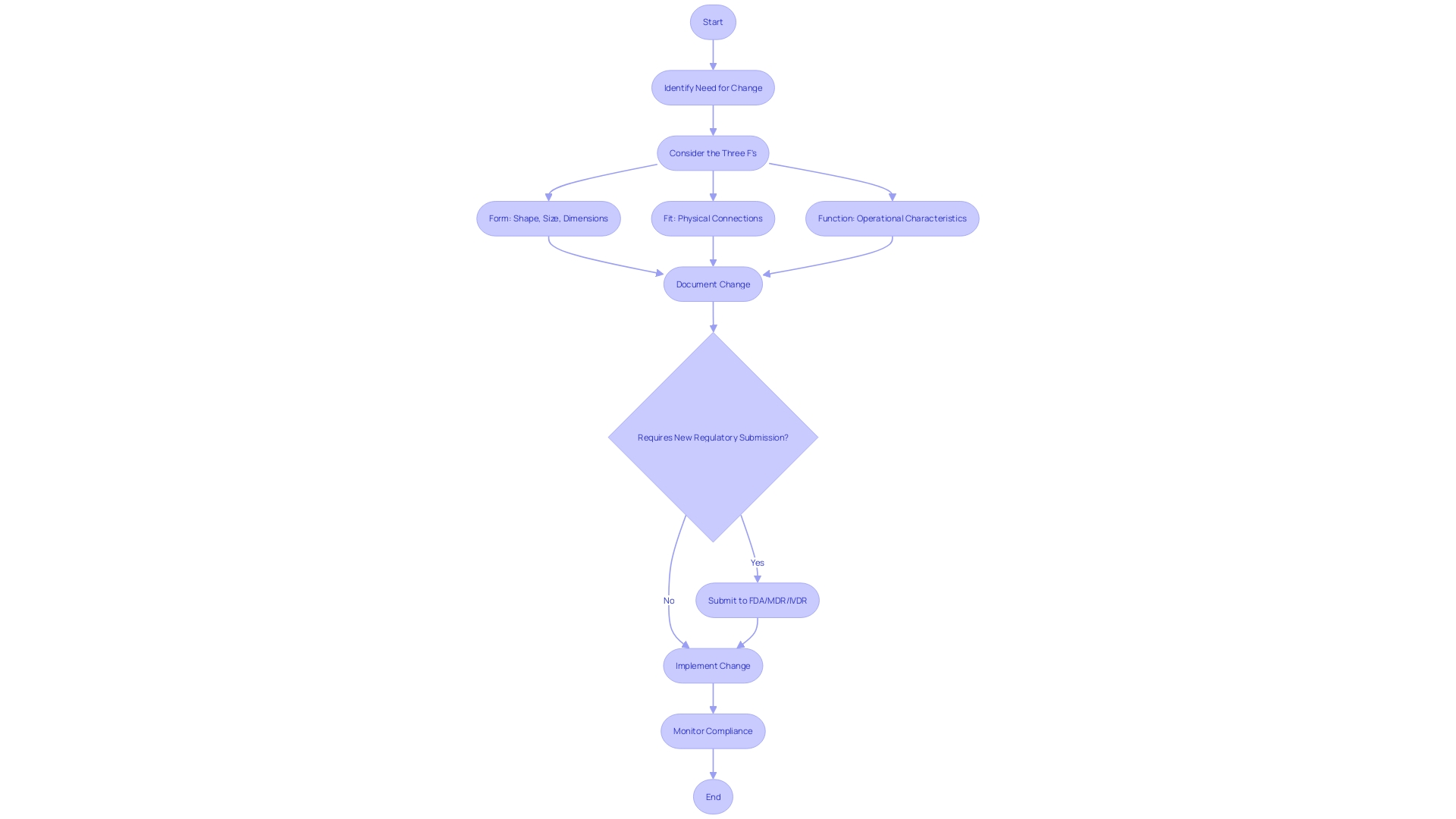
The changing legal framework, especially with the growth of Software as a Medical Device (SaMD), presents additional challenges. As demand for digital health solutions surges, understanding regional nuances and documentation requirements becomes essential. Experts are utilizing automated validation methods to enhance compliance, as promoted by oversight organizations like the FDA, which seeks to lessen dependence on manual paper-based systems.
Industry experts emphasize the importance of adapting to these changes to maintain compliance and efficiency. 'Practical methods, such as enhancing efficiency in compliance and safety document preparation, have been emphasized by experts maneuvering through this intricate landscape.'. These strategies not only minimize delays and errors but also address the intricacies of packaging and labeling, ensuring both compliance and product integrity.
James Pink, Senior Director at Element Materials Technology, emphasizes the importance of enhancing the safety and performance of healthcare products through advanced software solutions. His extensive experience in healthcare technology product safety and Regulatory Affairs underscores the ongoing efforts to lessen Regulatory burdens while ensuring high standards of product excellence and patient safety.
Key Components of Quality Management Systems
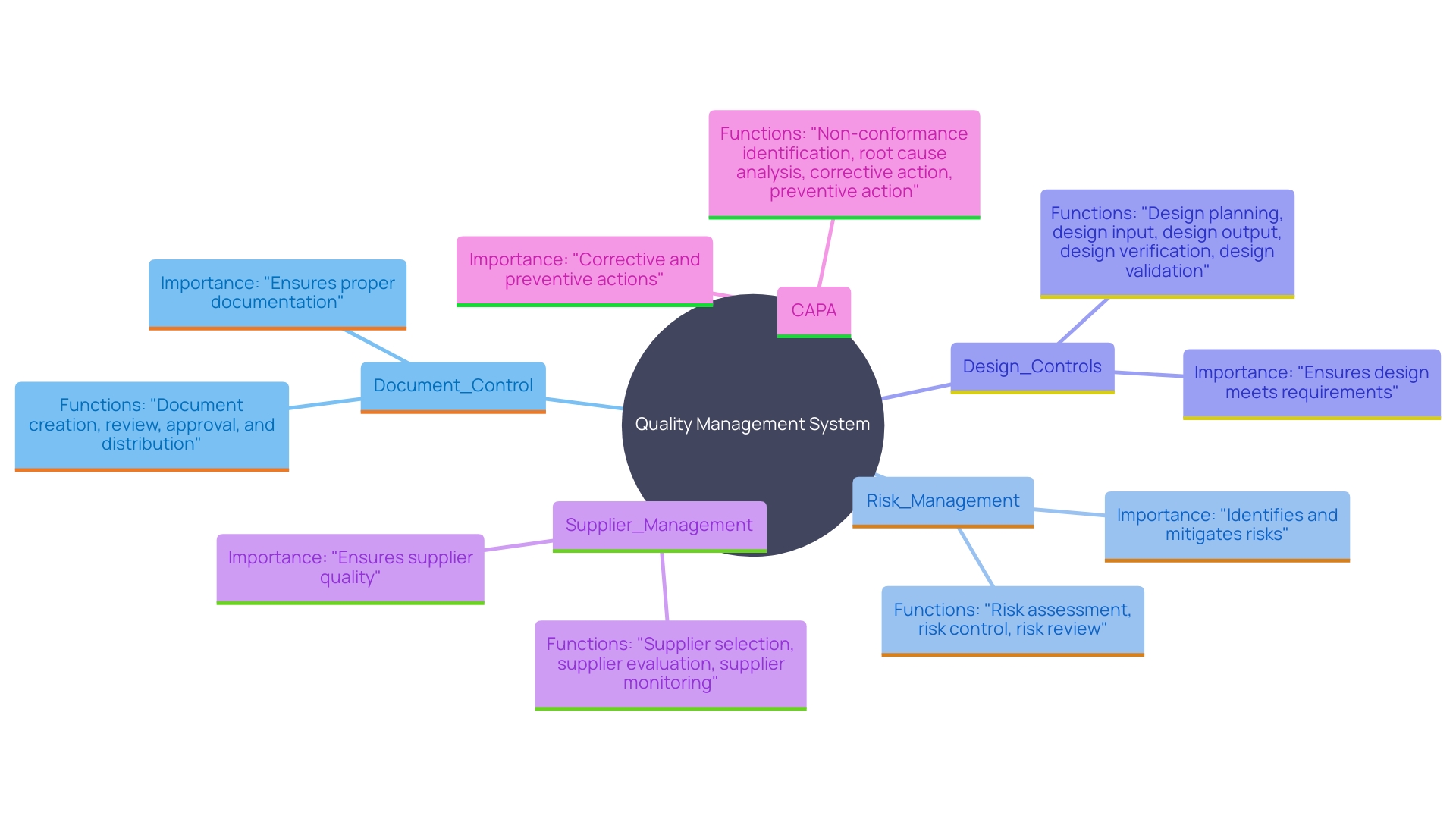
A robust quality management system in medical equipment projects encompasses essential components, each serving a vital function to ensure compliance and performance excellence. Document Control maintains all documentation current and accessible, a critical step given the variety of reasons changes might be necessary during a device's market life, such as customer feedback or material changes. Risk Management proactively identifies potential issues, safeguarding the project from unforeseen complications. Design Controls supervise the development process, requiring thorough testing and validation to meet strict compliance standards. Supplier Management involves rigorous assessment and maintenance of quality standards among vendors, ensuring the reliability of external contributions. Ultimately, Corrective and Preventive Actions (CAPA) tackle non-conformities, encouraging ongoing enhancement and compliance with legal requirements. 'As governing organizations like the FDA promote more automated methods, such as utilizing computer systems for validation, these elements together maintain the integrity, safety, and efficacy of medical devices.'.
Audits and Continuous Improvement
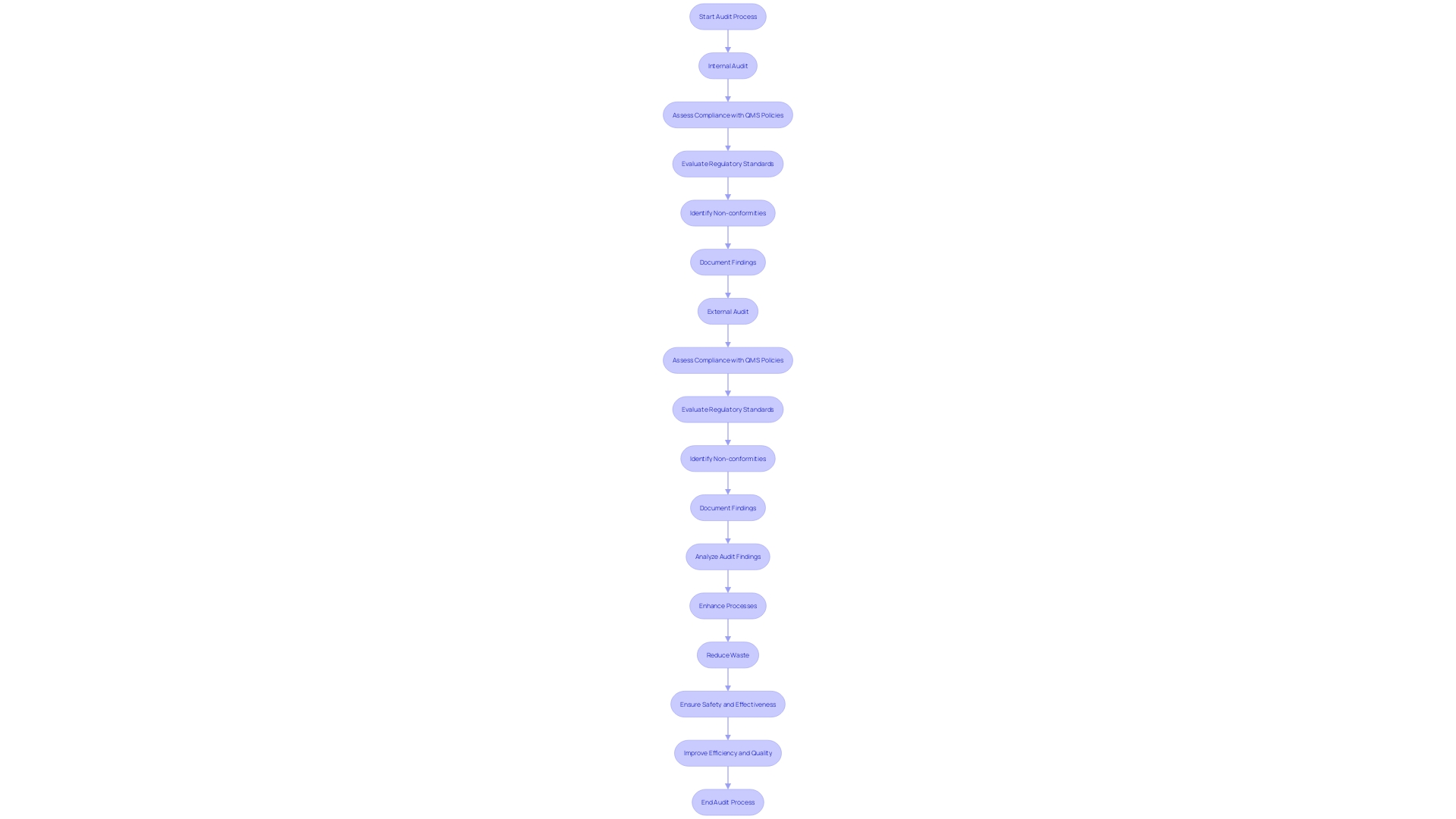
Carrying out regular audits is essential for ensuring compliance with standards and finding opportunities for enhancement. Internal audits allow organizations to evaluate their compliance with Quality Management System (QMS) policies, while external audits by regulatory bodies or certifying agencies ensure that operations align with relevant regulations. This dual approach not only encourages adherence but also promotes ongoing enhancement—a fundamental principle of management. By focusing on enhancing processes, reducing waste, and increasing efficiency, organizations can create a robust culture of quality. This commitment assists in ensuring that health instruments fulfill rigorous safety and effectiveness criteria, a requirement emphasized by the international oversight framework. In the medical device industry, compliance with standards such as ISO 13485 and FDA regulations is not merely a bureaucratic requirement; it is essential for ensuring patient safety and product reliability. An effective audit strategy can streamline the qualification process, overcoming challenges such as complex compliance requirements and confidentiality concerns. By integrating these audits into a broader quality management framework, organizations can better navigate the evolving regulatory demands and maintain high standards of product quality and performance.
Ensuring Safety Throughout the Device Lifecycle
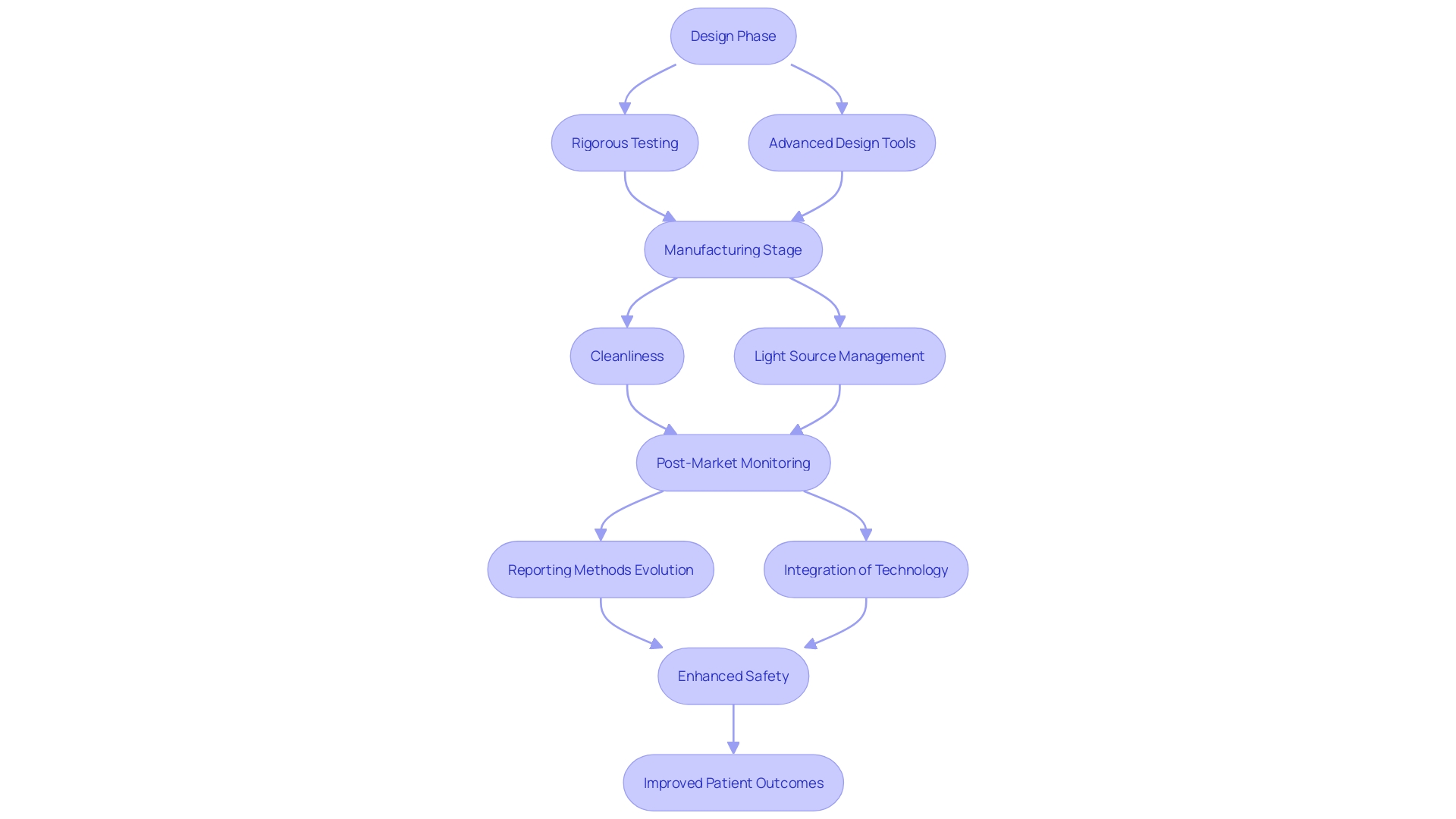
'Safety is paramount in medical equipment development, and it must be prioritized at every stage of the product lifecycle.'. This includes rigorous testing during the design phase, comprehensive evaluation during manufacturing, and ongoing monitoring post-launch. In the design phase, employing first principles or optical design software is crucial for understanding relevant variables and optimizing success of the apparatus. Key considerations span multiple domains, including electrical, mechanical, and software development, ensuring a holistic approach.
During manufacturing, cleanliness requirements tailored to the environment and application are essential to maintain safety standards. Light source variables, such as wavelength and power, must be meticulously managed to ensure proper operation and safety.
Post-market monitoring and vigilance reporting are essential for recognizing and reducing risks related to product use. Traditional methods of post-market monitoring, primarily relying on voluntary reporting, are evolving with technological advancements. Advanced healthcare equipment software, linked systems, and groundbreaking sensors are revolutionizing post-market intelligence, providing new chances to improve safety and performance.
By implementing thorough safety protocols at each stage, quality engineers can significantly reduce the likelihood of adverse events, ensuring robust patient safety. 'Bijan Elahi, a specialist in safety risk management for healthcare tools, highlights the significance of clarifying risk management to offer understanding and assurance to practitioners.'. This thorough method to safety can ultimately lead to improved patient results and more dependable health instruments.
Role of Quality Engineers
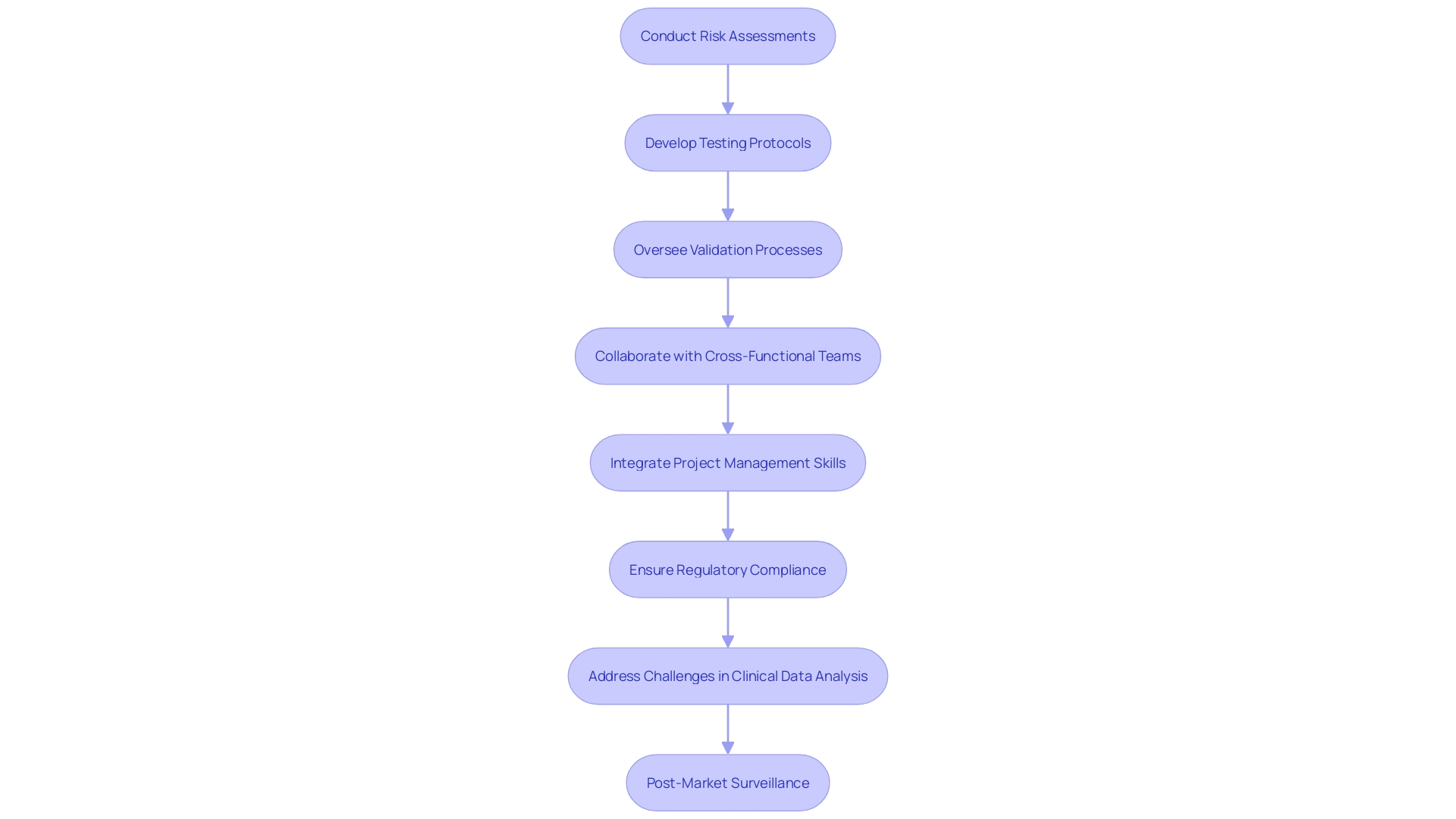
Quality engineers are essential in the development and management of medical equipment. They ensure regulatory compliance and adherence to standards, essential for the safety and efficacy of these products. Their responsibilities encompass conducting thorough risk assessments, developing rigorous testing protocols, and overseeing validation processes to maintain high standards throughout the product lifecycle.
Engineers focus intensely with cross-functional teams to incorporate standards into every stage of product development, from initial concept to market launch. This collaboration helps address potential risks early, ensuring that safety and effectiveness are built into the product from the outset.
A significant challenge in this role is the meticulous analysis and presentation of clinical data, which often lacks detailed coherence, leading to inconsistencies in documents such as clinical evaluation plans and risk management files. Post-market surveillance data, crucial for continuous improvement, is frequently underutilized, despite its importance in clinical evaluations.
Successful assurance of standards is not just about adherence but about providing healthcare products that consistently achieve high criteria and enhance patient results. As Jennifer Mascioli-Tudor aptly puts it, “A really good quality and regulatory professional needs to have really good project management skills.” This underscores the importance of integrating project management with regulatory expertise to navigate the complex landscape of medical device development successfully.
Conclusion
Quality management in medical devices is essential for ensuring compliance with safety and efficacy standards throughout the product lifecycle. Effective quality management systems (QMS), particularly those guided by ISO 13485, cover critical activities from design to post-market surveillance. Key elements such as document control, risk management, and continuous improvement are vital for maintaining quality.
Navigating the complex regulatory landscape, including FDA and European MDR pathways, is crucial for market entry. The shift toward automated validation processes enhances compliance and efficiency, especially with the rise of Software as a Medical Device (SaMD).
Regular internal and external audits are necessary to uphold quality standards and promote a culture of continuous improvement. Safety must be prioritized at every stage, leveraging advanced technologies for effective risk monitoring.
Quality engineers are integral to this process, ensuring regulatory compliance and collaborating across teams to embed quality considerations throughout development. Their expertise in managing clinical data and post-market surveillance is essential for achieving optimal patient outcomes.
Overall, a strong commitment to quality management is vital for ensuring the safety and reliability of medical devices, ultimately protecting public health in a highly regulated industry.




