Overview
The article focuses on effective strategies for leveraging real-world evidence (RWE) from clinical trials in Peru to enhance medical research and patient care. It emphasizes the importance of:
- Utilizing electronic health records
- Engaging local healthcare providers
- Addressing challenges such as data quality and regulatory compliance
These elements collectively support the successful integration of RWE into clinical studies, thereby improving treatment outcomes. The insights provided are crucial for understanding the Medtech landscape and the role of bioaccess in overcoming key challenges. Ultimately, collaboration is essential for driving progress in clinical research.
Introduction
In the evolving landscape of clinical research, real-world evidence (RWE) has emerged as a powerful tool that complements traditional methodologies, offering a fresh lens through which to evaluate the effectiveness and safety of medical interventions.
Particularly in regions like Peru, where diverse demographics and healthcare practices can significantly shape treatment outcomes, the integration of RWE into clinical trials is not just beneficial—it is essential.
This article delves into the critical importance of RWE in enhancing the generalizability of research findings, addressing health inequities, and informing regulatory decisions.
By exploring the strategies for collecting and utilizing RWE, along with the challenges researchers face, it becomes clear that leveraging real-world insights is key to transforming healthcare and improving patient care in the modern age.
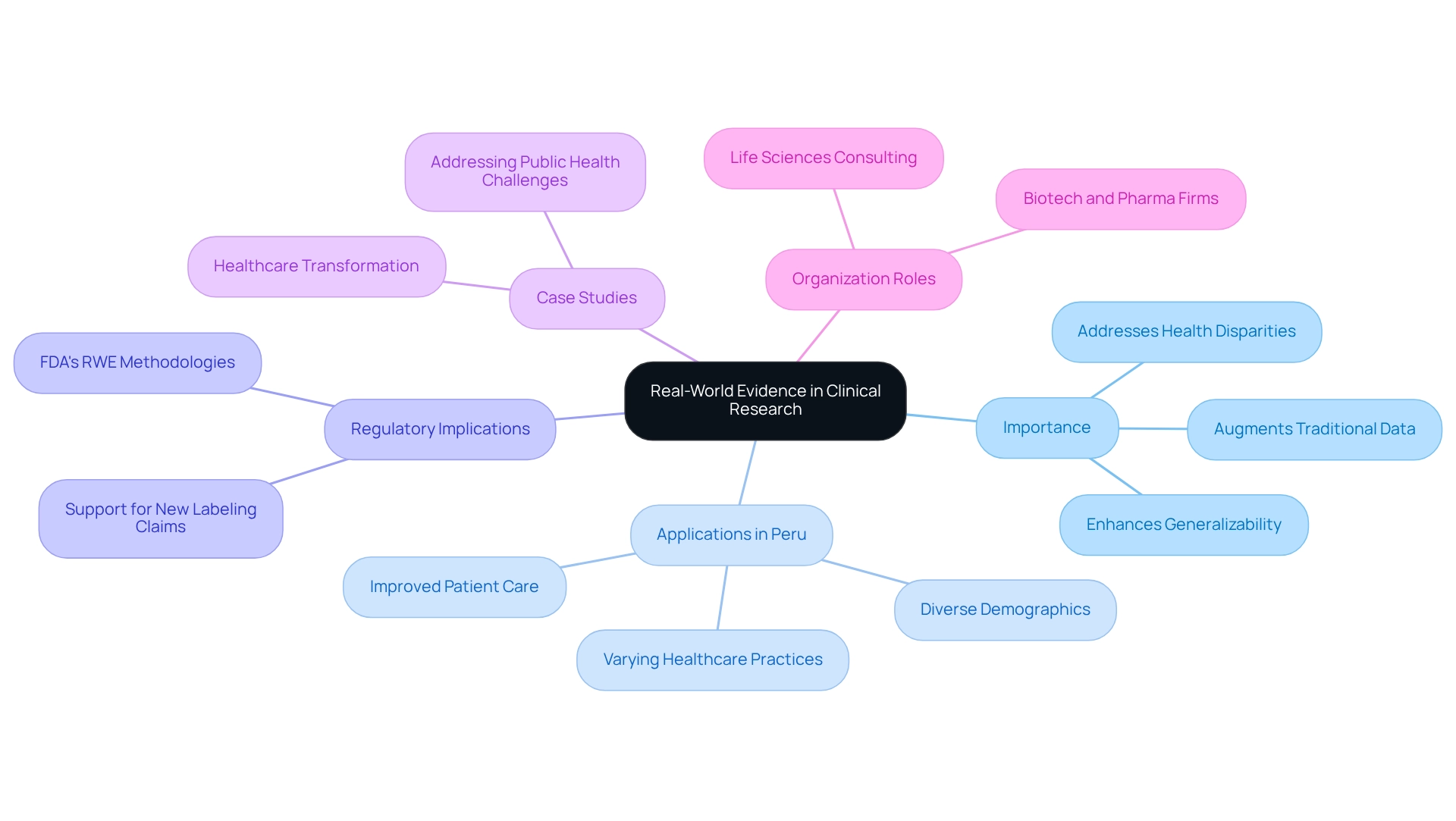
Understand Real-World Evidence and Its Importance in Clinical Research
Real-world evidence (RWE) encompasses practical insights derived from real-world data (RWD) regarding the usage, advantages, and risks associated with medical products. Its growing recognition in clinical research stems from its ability to augment traditional clinical study data, offering crucial insights into treatment efficacy, safety, and patient outcomes in everyday healthcare settings. Notably, RWE is vital in identifying populations that may be underrepresented in randomized controlled trials (RCTs), thereby enhancing the generalizability of research findings.
This relevance is particularly pronounced in Peru, where real-world evidence from Peruvian trials indicates that diverse demographics and varying healthcare practices can significantly influence treatment outcomes. By harnessing RWE, researchers can design studies that more accurately mirror real-world scenarios, ultimately fostering improved patient care and more informed regulatory decisions. The integration of RWE into medical studies not only addresses health disparities but also aligns with current trends that emphasize the importance of real-world data in advancing medical research and treatment accessibility. As highlighted, the real-world evidence solutions market in Japan commanded a substantial market share in 2023, underscoring the global significance of RWE.
Moreover, the FDA has articulated, "This program aims to enhance the quality and acceptance of RWE-based methodologies for supporting new labeling claims, including approving new applications for existing medical products or meeting post-approval study obligations." This authoritative statement reinforces the critical role of RWE in regulatory decision-making.
Additionally, a case study titled "The Role of Real-World Evidence in Healthcare Transformation" exemplifies how RWE is increasingly recognized for its potential to revolutionize healthcare by tackling issues such as health inequities and public health challenges, including the COVID-19 pandemic. The urgency for biotech, pharmaceutical, life sciences, and biopharma consulting firms to adopt RWE to maintain competitiveness in the industry further highlights the significance of RWE in today’s research landscape.
At bioaccess®, we provide comprehensive management services for studies, encompassing feasibility assessments, site selection, compliance evaluations, setup, import permits, project oversight, and reporting. Our expertise ensures that the integration of real-world evidence from Peruvian trials into medical studies is executed efficiently, which enhances the quality of care and maximizes outcomes for individuals across Latin America.
Explore the Landscape of Clinical Trials in Peru
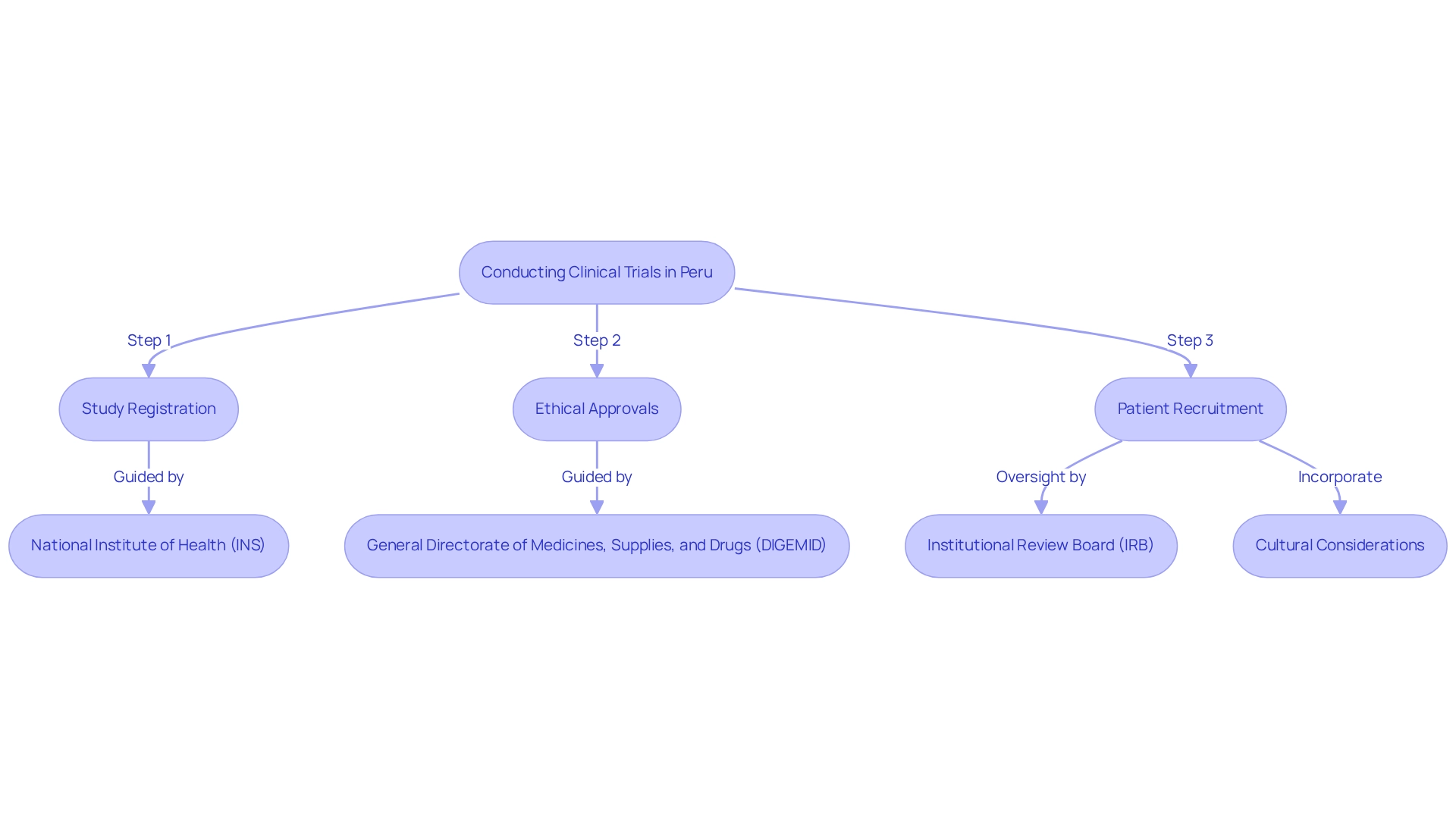
Peru has established itself as a vital player in the research landscape, with real-world evidence from Peruvian trials, significant regulatory advancements, and an increasing willingness among patients to participate in studies. The National Institute of Health (INS) oversees the regulatory framework, ensuring that research studies adhere to international standards while catering to local needs. As of early 2025, more than 1,200 clinical studies have been registered in Peru, which serve as real-world evidence from Peruvian trials and reflect a strong interest across various therapeutic domains, particularly within the Medtech sector. Notably, between 2005 and 2022, the FDA granted its first premarket approvals for several medtech products, signaling a growing trend in regulatory acceptance that aligns with the progress observed in Peru.
Researchers are required to navigate specific regulatory protocols, including study registration and ethical approvals, which are critical for the successful implementation of studies. The General Directorate of Medicines, Supplies, and Drugs (DIGEMID) plays a pivotal role in this process, offering binding opinions that shape approval pathways based on safety evaluations as stipulated in the Regulation of Clinical Trials established by Supreme Decree No 021-2017-SA. This regulatory framework is further reinforced by the Institutional Review Board (IRB), a non-profit entity that protects the rights and well-being of research participants, ensuring ethical compliance throughout the research endeavor, which is supported by real-world evidence from Peruvian trials.
Additionally, cultural factors and patient demographics significantly influence recruitment strategies and data collection methodologies. Grasping these elements is essential for researchers to effectively customize their approaches, ensuring they engage with the local population in a manner that honors cultural sensitivities and boosts participation rates. This adaptability is crucial for leveraging the unique opportunities presented by the Peruvian research environment.
Furthermore, bioaccess® offers comprehensive management services for studies, including feasibility assessments, site selection, compliance evaluations, setup, import permits, project oversight, and reporting on both serious and non-serious adverse events, all of which are vital for navigating the intricacies of research in this region. The analysis of the regulatory framework for research studies in Peru illustrates how DIGEMID's evaluations shape the approval process and foster a conducive environment for medical research. Connect with bioaccess® to learn more about how we can assist with your research needs.
Implement Strategies for Collecting and Utilizing RWE from Peruvian Trials
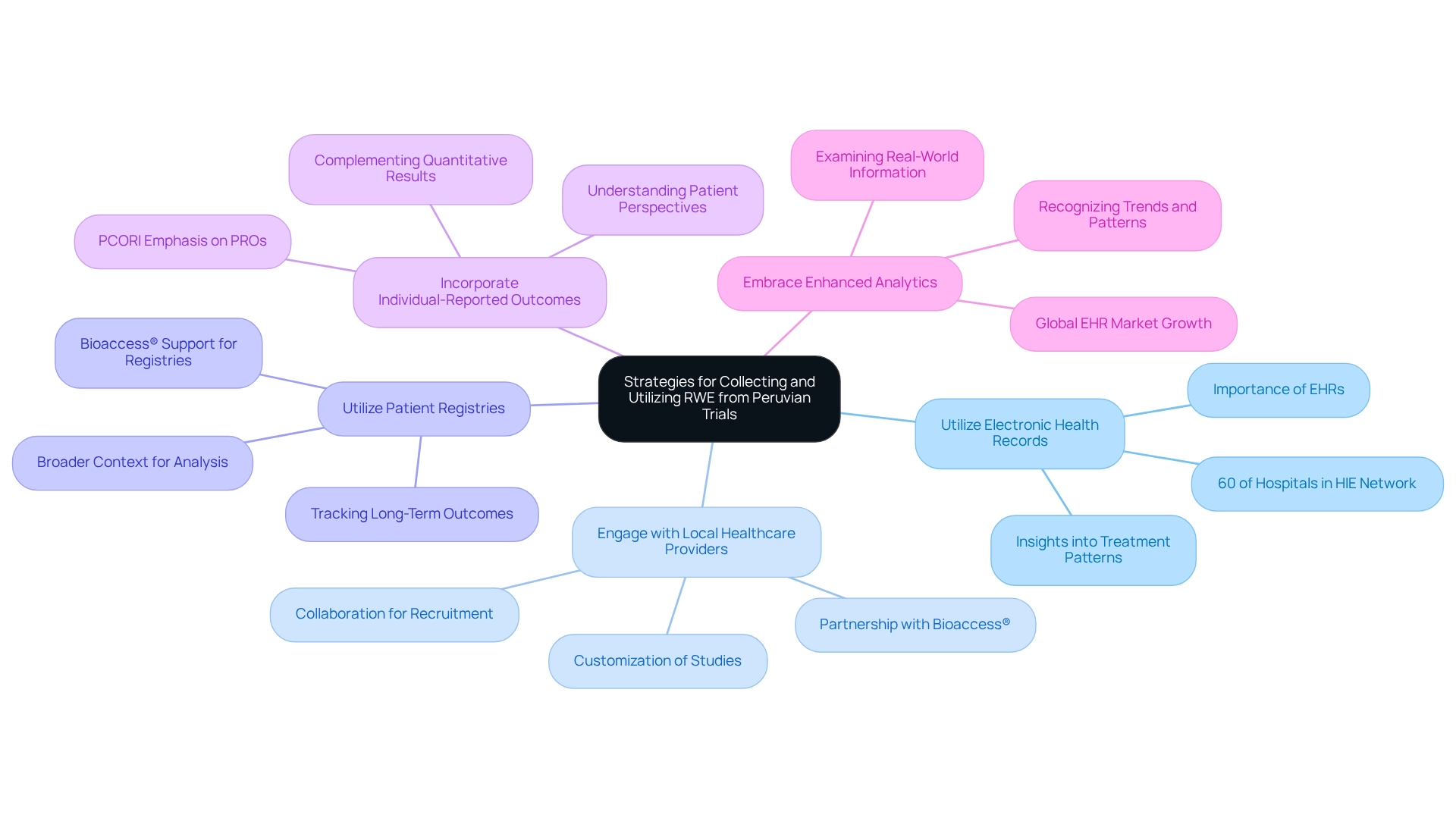
To effectively gather and utilize real-world evidence (RWE) from research studies in Peru, researchers should implement the following strategies:
- Utilize Electronic Health Records (EHRs): EHRs serve as a vital asset for collecting extensive individual information, including demographics, treatment histories, and outcomes. With 60% of hospitals participating in a Health Information Exchange (HIE) network, the infrastructure for EHRs in Peru is expanding, supporting the strategies outlined for leveraging EHRs in RWE collection. This information provides valuable insights into real-world treatment patterns and their effectiveness, which is supported by real-world evidence from Peruvian trials, thereby enhancing the overall quality of clinical research.
- Engage with Local Healthcare Providers: Collaborating with local physicians and healthcare institutions is essential for facilitating participant recruitment and data collection. Their expertise assists in customizing studies, including Early-Feasibility Studies (EFS) and First-In-Human Studies (FIH), to better align with local practices and address specific individual needs, ultimately enhancing the relevance of the findings. Partnering with bioaccess® can further strengthen this collaboration, as they bring over 20 years of experience in managing clinical trials across Latin America.
- Utilize Patient Registries: Establishing or accessing existing patient registries enables researchers to track long-term outcomes and treatment responses. This approach enriches the information gathered and provides a broader context for analysis, leading to more substantial conclusions. Bioaccess® can assist in establishing these registries, ensuring compliance and efficient information management throughout the study.
- Incorporate Individual-Reported Outcomes (PROs): Collecting PROs is crucial for understanding individuals' perspectives on treatment effectiveness and quality of life. As emphasized by the Patient-Centered Outcomes Research Institute (PCORI), this qualitative information complements quantitative results, offering a more comprehensive view of treatment effects and enhancing the overall understanding of individual experiences. Bioaccess® underscores the significance of PROs in their research management services, ensuring that patient voices are acknowledged.
- Embrace Enhanced Analytics: Utilizing analytical tools allows researchers to examine real-world information proficiently, recognizing trends and patterns that can guide medical decision-making and regulatory submissions. The global EHR market is anticipated to expand at a CAGR of 6.8% from 2023 to 2028, highlighting the broader context of EHR uptake and its implications for research. This analytical approach is crucial for transforming information into actionable insights that can elevate patient care. With bioaccess®'s expertise in project management and reporting, researchers can leverage advanced analytics to improve their studies' outcomes and contribute to local economic growth through enhanced healthcare solutions, including job creation and healthcare improvement.
Address Challenges and Solutions in Leveraging RWE
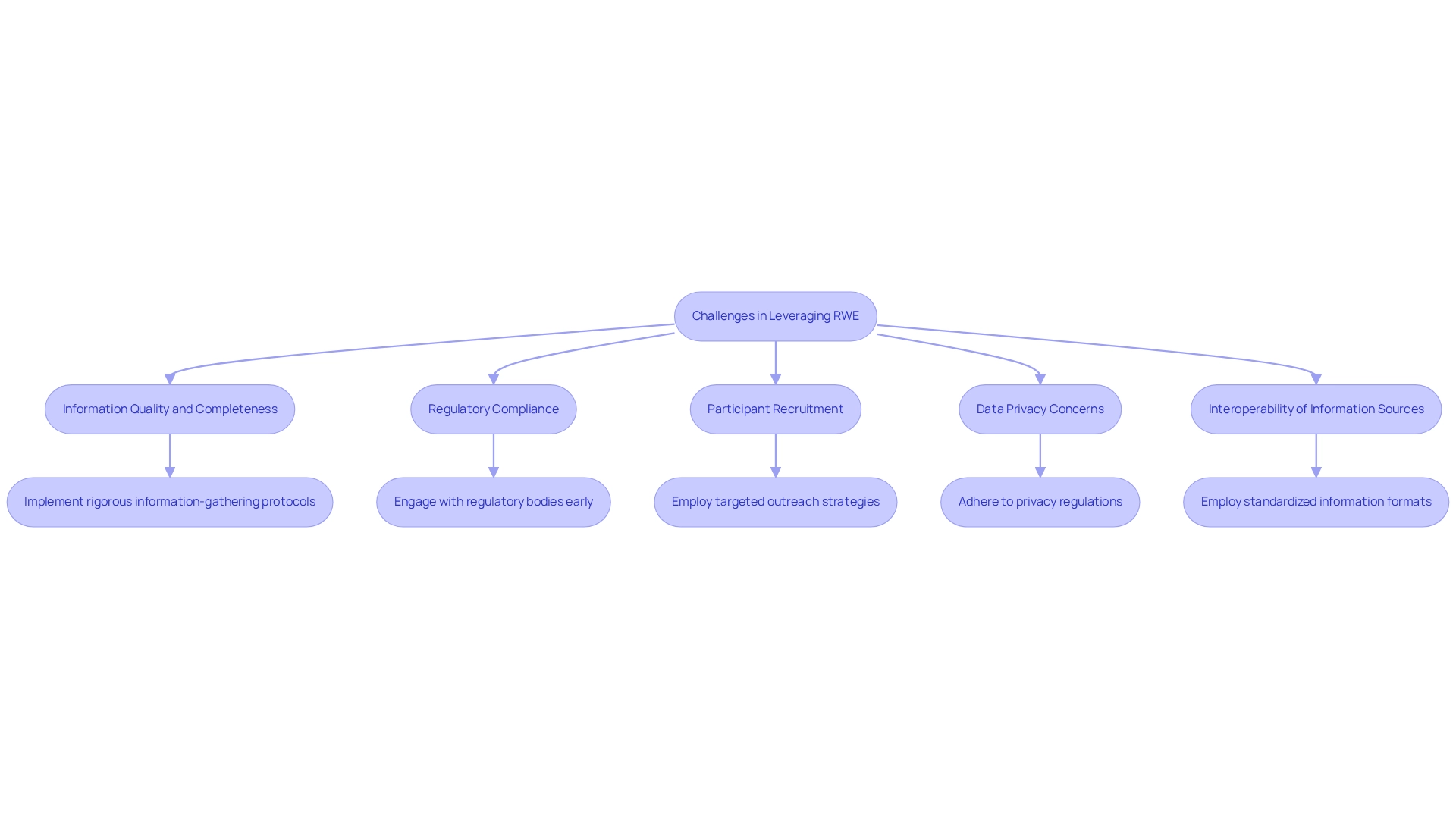
Leveraging real-world evidence (RWE) in clinical trials presents significant opportunities; however, researchers encounter several challenges that must be effectively addressed:
-
Information Quality and Completeness: High-quality information is essential for reliable outcomes. Implementing rigorous information-gathering protocols and validation processes is crucial to minimize errors and biases, ensuring that the information collected is both precise and comprehensive. As noted by Lauren Houston, survey results indicate heterogeneity within and between organizational types, including academic/government, clinical research organizations, and industry, which can impact data quality.
-
Regulatory Compliance: The regulatory landscape can be intricate and varies by region. Engaging with regulatory bodies early in the study design process clarifies requirements and streamlines the approval process, reducing delays and enhancing compliance. Bioaccess excels in this area by offering comprehensive services that include review and feedback on study documents to meet country requirements, ensuring that healthcare organizations utilize advanced analytics to uphold compliance while improving care.
-
Participant Recruitment: Achieving a varied participant population frequently poses a considerable challenge. Employing targeted outreach strategies and collaborating with local healthcare providers can enhance recruitment efforts, ensuring that the study population reflects the broader community.
-
Data Privacy Concerns: Safeguarding individual privacy is of utmost importance. Researchers must adhere to local and global privacy regulations, ensuring ethical management of patient information and preserving trust throughout the research process.
-
Interoperability of Information Sources: Combining information from various origins can present difficulties. Employing standardized information formats and platforms promotes smoother integration and analysis of information, improving the overall quality of the research.
Most survey participants indicated that staff training and development were conducted, emphasizing the significance of training in guaranteeing information quality and regulatory compliance. By proactively tackling these challenges, researchers can significantly improve the effectiveness of real-world evidence from Peruvian trials in their studies, leading to more robust and impactful findings. For instance, advanced data management approaches have been shown to reduce drug development cycles by an average of 1.5 years and increase the likelihood of regulatory approval by 23%, underscoring the importance of effective data strategies in clinical research.
Conclusion
Real-world evidence (RWE) transcends being a mere addition to clinical research; it serves as a transformative force that enhances the relevance and applicability of study findings, particularly in diverse healthcare settings like Peru. By integrating RWE, researchers can effectively address health inequities, improve patient outcomes, and inform regulatory decisions. This ensures that clinical trials reflect the realities of patient experiences and treatment effectiveness in everyday practice.
The strategies for collecting and utilizing RWE—leveraging electronic health records, engaging local healthcare providers, and incorporating patient-reported outcomes—underscore the multifaceted approach essential for harnessing this valuable data. Despite challenges such as data quality, regulatory compliance, and patient recruitment, proactive solutions can significantly mitigate these obstacles, enabling researchers to maximize the potential of RWE in their studies.
Ultimately, the commitment to integrating real-world insights into clinical trials is crucial for advancing healthcare and enhancing patient care across Latin America. As the landscape of clinical research continues to evolve, embracing RWE will be pivotal in driving innovation and ensuring that medical interventions are not only effective in controlled environments but also resonate with the diverse populations they aim to serve.
Frequently Asked Questions
What is real-world evidence (RWE)?
Real-world evidence (RWE) consists of practical insights derived from real-world data (RWD) regarding the usage, advantages, and risks associated with medical products.
Why is RWE important in clinical research?
RWE is important because it augments traditional clinical study data, providing crucial insights into treatment efficacy, safety, and patient outcomes in everyday healthcare settings.
How does RWE enhance the generalizability of research findings?
RWE helps identify populations that may be underrepresented in randomized controlled trials (RCTs), thereby enhancing the applicability of research findings to a broader patient population.
What is the relevance of RWE in Peru?
In Peru, RWE from trials shows that diverse demographics and varying healthcare practices can significantly influence treatment outcomes, allowing researchers to design studies that reflect real-world scenarios.
How does RWE contribute to patient care and regulatory decisions?
By integrating RWE into medical studies, researchers can improve patient care and make more informed regulatory decisions, addressing health disparities and aligning with trends in advancing medical research and treatment accessibility.
What statement did the FDA make regarding RWE?
The FDA stated that their program aims to enhance the quality and acceptance of RWE-based methodologies for supporting new labeling claims and for approving new applications for existing medical products or meeting post-approval study obligations.
What role does RWE play in healthcare transformation?
RWE is increasingly recognized for its potential to revolutionize healthcare by addressing issues such as health inequities and public health challenges, including the COVID-19 pandemic.
Why is it important for biotech and pharmaceutical firms to adopt RWE?
The urgency for these firms to adopt RWE is highlighted by the need to maintain competitiveness in the industry, emphasizing RWE's significance in today's research landscape.
What services does bioaccess® provide in relation to RWE?
Bioaccess® offers comprehensive management services for studies, including feasibility assessments, site selection, compliance evaluations, setup, import permits, project oversight, and reporting, ensuring efficient integration of RWE from Peruvian trials into medical studies.




