Overview
Real-world evidence (RWE) in Chilean trials plays a critical role in understanding treatment efficacy within everyday settings. However, its implementation is hindered by challenges, including data quality and regulatory compliance. This article underscores the necessity of robust data collection strategies and active engagement with local stakeholders to bolster RWE's credibility and effectiveness in guiding healthcare decisions. It highlights the urgent need for standardized practices and collaboration to effectively tackle these challenges.
Introduction
In the evolving landscape of healthcare, the integration of Real-World Evidence (RWE) is transforming the conduct and evaluation of clinical trials. By harnessing data collected outside traditional clinical settings, RWE offers invaluable insights into treatment effectiveness and patient outcomes, thereby enhancing decision-making processes and regulatory approvals.
As the global market for RWE solutions continues to expand, it becomes increasingly vital to understand its implications and the challenges faced—particularly in regions like Chile. This article delves into the significance of RWE in clinical trials, explores the regulatory frameworks governing these studies, and identifies effective strategies for data collection and patient engagement, while addressing the obstacles that researchers encounter in implementing RWE.
Through a comprehensive examination of these elements, the article highlights the potential of RWE to revolutionize patient care and improve health outcomes in a real-world context.
Define Real-World Evidence and Its Importance in Clinical Trials
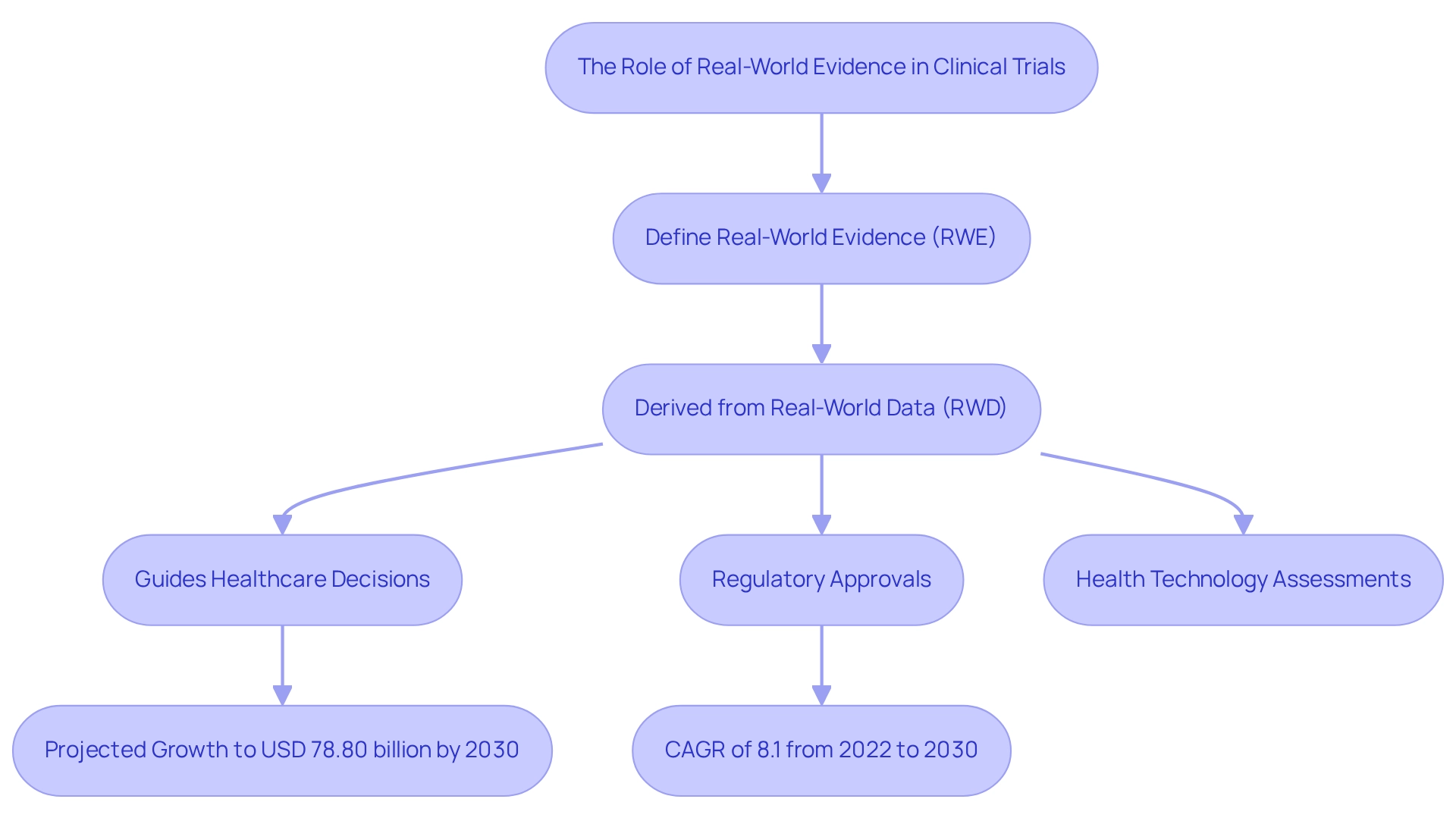
Real-World Evidence (RWE) is derived from the analysis of Real-World Data (RWD), encompassing information collected beyond the confines of traditional trials. RWE is crucial in trials, providing insights into treatment efficacy in real-life settings, capturing vital data on outcomes, treatment adherence, and long-term effects. Its significance is highlighted by the anticipated growth of the global RWE solutions market, projected to reach USD 78.80 billion by 2030. This reflects a shift towards value-based care and the increasing acknowledgment of RWE's role in healthcare. Furthermore, the market for real-world evidence solutions is expected to expand at a compound annual growth rate (CAGR) of 8.1 percent from 2022 to 2030, indicating robust growth dynamics in this sector.
RWE is instrumental in guiding healthcare decisions, regulatory approvals, and health technology assessments, ultimately enhancing care and outcomes. For instance, it can reveal the effectiveness of treatments across diverse populations that may not be adequately represented in randomized controlled trials (RCTs). Engaging local stakeholders is essential for optimizing RWE implementation, as recent findings underscore the need for collaboration in this domain. Additionally, the European Medicines Agency (EMA) is establishing a digital innovation lab and developing comprehensive guidelines for AI application in regulatory processes, emphasizing the evolving landscape of healthcare decision-making.
As the healthcare environment evolves, the integration of RWE into clinical trials will become increasingly vital, enabling more informed decision-making and improved outcomes for individuals. Future research may be necessary to uncover new perspectives on RWE implementation, ensuring that practices continue to progress. A notable example is the NHS, which possesses an extensive repository of healthcare information that could be harnessed for RWE. By effectively utilizing its information resources, the NHS could accelerate the development and distribution of safe, affordable medicines, benefiting both patients and the healthcare system. This illustrates how efficient data utilization can lead to enhanced healthcare outcomes, demonstrating a practical application of RWE in a real-world context.
Explore the Regulatory Framework for Clinical Trials in Chile
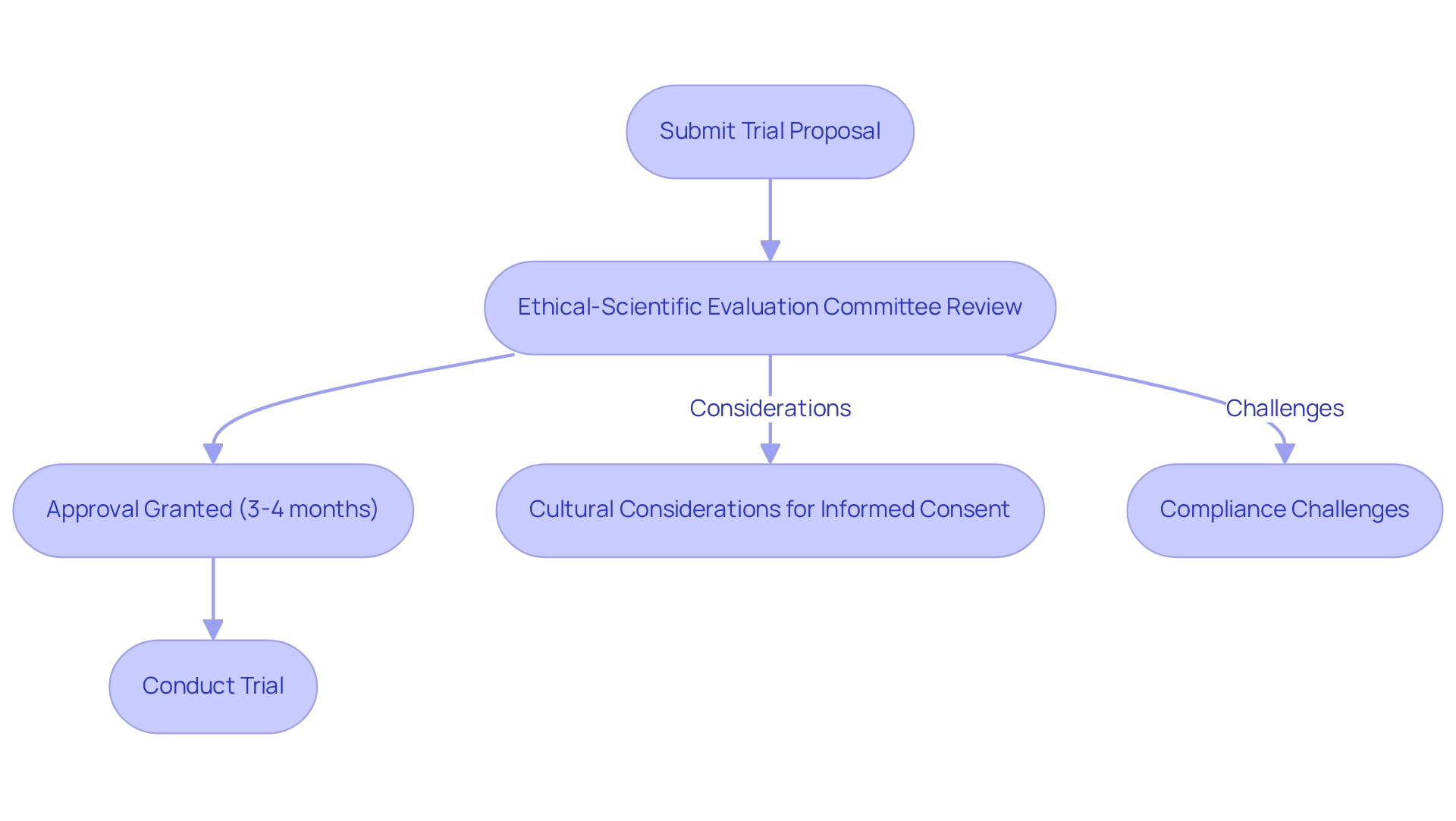
In Chile, the regulatory framework governing research trials is predominantly overseen by the Ministry of Health and the Public Health Institute (ISP). Clinical trials necessitate approval from an Ethical-Scientific Evaluation Committee (EC), which meticulously assesses both the ethical implications and scientific validity of proposed studies. The average approval duration for trials in Chile spans approximately 3-4 months, positioning the nation as a relatively efficient environment for conducting research. Nevertheless, navigating these regulations can pose challenges, particularly regarding compliance with local laws, including informed consent and data protection regulations.
Cultural differences across Latin America significantly influence informed consent procedures, as patients frequently accept physician recommendations without question. This dynamic can result in misunderstandings regarding participation in research studies, making it imperative for researchers to navigate these cultural nuances while adhering to ethical standards. As highlighted by Maryam Kermanimojarad, this research provides policy-makers with fresh insights into the development challenges facing the pharmaceutical sector, underscoring the importance of understanding the regulatory framework.
Furthermore, the introduction of new regulatory safeguards in Chile has impacted the number of trials focused on diseases with a high burden, reflecting the evolving landscape of research in the country. Effectively navigating these regulations is crucial for researchers committed to maintaining ethical standards while advancing their studies. By leveraging comprehensive clinical trial management services, such as those provided by bioaccess®, researchers can streamline this process, ensuring studies are conducted efficiently and ethically. This ultimately fosters global health improvement through international collaboration and innovation in Medtech.
Implement Strategies for Effective RWE Data Collection and Patient Engagement
To effectively gather Real-World Evidence (RWE), researchers must adopt a multi-faceted strategy that incorporates both prospective and retrospective information collection methods. By utilizing electronic health records, registries, and mobile health applications, researchers can facilitate the comprehensive collection of data on outcomes. Involving individuals throughout the research process is vital for improving participation and ensuring that their viewpoints are taken into account. Approaches such as:
- Partnering with advocacy organizations
- Conducting surveys to assess needs
- Employing technology for immediate feedback
can significantly enhance involvement.
For instance, incorporating participant-reported outcomes (PROs) into study designs not only enriches the information gathered but also provides essential insights into treatment effectiveness and quality of life from the participant's perspective. Notably, studies indicate that when both patients and providers have access to shared analytics dashboards, patient-provider satisfaction can increase by 31%, underscoring the value of transparency and collaboration in clinical research. However, it is crucial for investigators to refrain from generalizing findings from Real-World Data (RWD) to groups they are uncertain about, as inadequate validation can lead to misleading or harmful predictions. The representativeness of RWD is critical for generalizing conclusions, and rigorous methodologies must be employed to ensure the credibility and reliability of real-world evidence in Chilean trials used in regulatory decisions, as emphasized by the FDA.
Furthermore, machine learning techniques are increasingly applied to RWD for predictive modeling and causal inference, showcasing the advantages of advanced analytics in generating real-world evidence in Chilean trials. This approach not only enhances the depth of analysis but also fosters a more nuanced understanding of the complexities involved in clinical research.

Identify Challenges and Solutions in Utilizing RWE in Chilean Trials
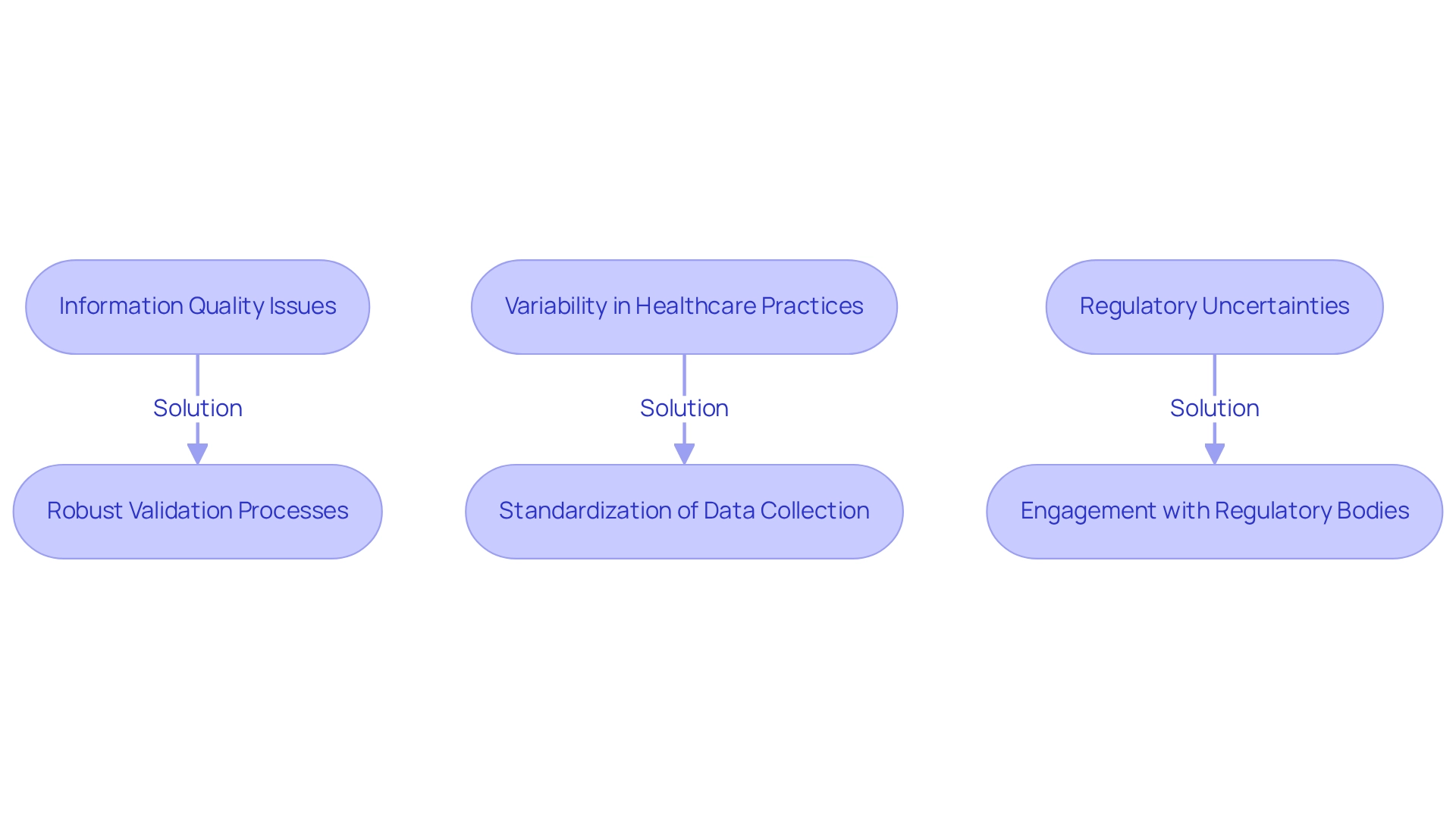
Employing real-world evidence in Chilean trials presents notable challenges, particularly concerning information quality, variability in healthcare practices, and regulatory uncertainties. A critical issue lies in the completeness and accuracy of Real-World Data (RWD), which can introduce biases that skew results. Reliability encompasses both the quality of the information points and the completeness of the data, underscoring the importance of robust validation processes.
To address these challenges, bioaccess offers comprehensive clinical trial management services, including feasibility studies and site selection, which are essential for ensuring thorough and representative information collection. Researchers should actively foster partnerships with local healthcare providers to enhance information gathering.
Furthermore, the variability in treatment practices across different regions complicates the interpretation of Real-World Evidence (RWE). Standardizing information collection methods and establishing clear protocols can help mitigate these discrepancies.
The case study titled "Challenges in Data Quality and Reliability" highlights significant concerns regarding the quality and reliability of real-world evidence, including issues related to completeness, accuracy, and potential biases. Improving data quality and establishing robust data governance frameworks are critical for enhancing the credibility of RWD and its integration into Health Technology Assessment (HTA) processes.
Staying informed about evolving regulations and engaging with regulatory bodies early in the study design process is vital for facilitating smoother approvals and bolstering the credibility of RWE findings. As Marshall H. Chin noted, moving away from 'fairness via unawareness' offers transparency and explicit measures of fairness that can be optimized alongside traditional objectives like predictive accuracy.
Addressing these challenges is essential for effectively leveraging real-world evidence in Chilean trials, ultimately leading to more reliable outcomes and insights.
Conclusion
The integration of Real-World Evidence (RWE) into clinical trials signifies a transformative shift in healthcare, offering essential insights into treatment effectiveness and patient experiences that traditional methods may overlook. By leveraging data from real-world environments, RWE deepens the understanding of patient outcomes and informs regulatory decisions, thereby fostering a more patient-centered approach to care.
In Chile, the regulatory framework governing clinical trials presents challenges, particularly concerning informed consent and cultural nuances. Nevertheless, the relatively efficient approval process provides an opportunity for researchers to collaborate with local stakeholders, optimizing the implementation of RWE and enhancing both the healthcare system and patient outcomes.
To effectively harness RWE, researchers must implement robust data collection and patient engagement strategies. Engaging patients and utilizing diverse data sources enriches the quality of the evidence gathered. Addressing challenges related to data quality and variability in healthcare practices is crucial for ensuring credible findings.
Ultimately, embracing RWE not only facilitates better decision-making but also leads to improved health outcomes in real-world settings. The potential for RWE to revolutionize clinical trials and enhance patient care is substantial, underscoring the necessity for ongoing collaboration and innovation in research practices. By integrating these insights into everyday healthcare, the industry can advance toward a more effective and responsive system that genuinely meets the needs of patients.
Frequently Asked Questions
What is Real-World Evidence (RWE)?
Real-World Evidence (RWE) is derived from the analysis of Real-World Data (RWD), which encompasses information collected outside of traditional clinical trials. It provides insights into treatment efficacy in real-life settings and captures important data on outcomes, treatment adherence, and long-term effects.
Why is RWE significant in healthcare?
RWE is crucial for guiding healthcare decisions, regulatory approvals, and health technology assessments. It helps enhance care and outcomes by revealing the effectiveness of treatments across diverse populations that may not be well-represented in randomized controlled trials (RCTs).
What is the projected growth of the global RWE solutions market?
The global RWE solutions market is projected to reach USD 78.80 billion by 2030, reflecting a shift towards value-based care and the increasing acknowledgment of RWE's role in healthcare.
What is the expected compound annual growth rate (CAGR) for the RWE market from 2022 to 2030?
The market for real-world evidence solutions is expected to expand at a compound annual growth rate (CAGR) of 8.1 percent from 2022 to 2030.
How can local stakeholders contribute to RWE implementation?
Engaging local stakeholders is essential for optimizing RWE implementation, as collaboration is necessary to effectively utilize real-world data and enhance healthcare outcomes.
What initiatives is the European Medicines Agency (EMA) taking regarding RWE?
The European Medicines Agency (EMA) is establishing a digital innovation lab and developing comprehensive guidelines for the application of artificial intelligence (AI) in regulatory processes, highlighting the evolving landscape of healthcare decision-making.
How does RWE integration into clinical trials benefit healthcare?
The integration of RWE into clinical trials enables more informed decision-making, ultimately leading to improved outcomes for individuals as the healthcare environment continues to evolve.
Can you provide an example of RWE application in practice?
A notable example is the NHS, which has an extensive repository of healthcare information that could be utilized for RWE. By effectively using its information resources, the NHS could accelerate the development and distribution of safe, affordable medicines, benefiting both patients and the healthcare system.




