Introduction
Navigating the regulatory landscape for medical devices in Brazil involves understanding the intricate framework overseen by the Brazilian Health Regulatory Agency (ANVISA). This agency is pivotal in ensuring that all medical devices comply with stringent safety and efficacy standards before entering the market. The regulatory framework, influenced by both national legislation and international standards, aims to protect public health while fostering innovation in the medical technology sector.
ANVISA's role extends beyond mere regulation; it actively promotes the advancement and standardization of medical technologies, ensuring that patients have access to safe and effective devices. The comprehensive assessment process, including clinical trials and post-market surveillance, underscores the agency's commitment to maintaining high standards. However, developers must also navigate challenges such as regulatory delays and import complexities, which can impact the timely execution of clinical trials.
Harmonizing Brazil's regulatory standards with international frameworks presents a pathway to streamline processes, enhance market access, and support innovation in the rapidly evolving MedTech sector.
Overview of Regulatory Framework
Brazil's oversight framework for healthcare instruments is managed mainly by the Brazilian Health Supervisory Agency (ANVISA). This agency is responsible for ensuring the safety and effectiveness of healthcare instruments in the Brazilian market. The regulatory framework is shaped by both national legislation and international standards, aiming to safeguard public health while fostering innovation in the healthcare technology sector. ANVISA plays a crucial role in fostering innovation and standardization in technologies that facilitate patient access to novel instruments. It provides leadership in quality standards and utilization through outreach and global harmonization. The agency guarantees that all health instruments comply with rigorous safety and effectiveness criteria, securing patient protection and alignment with global standards. This governing environment is designed to balance the need for rigorous safety protocols with the promotion of technological advancements, thus driving the evolution of the MedTech sector in Brazil.
ANVISA's Role in Medical Device Regulation
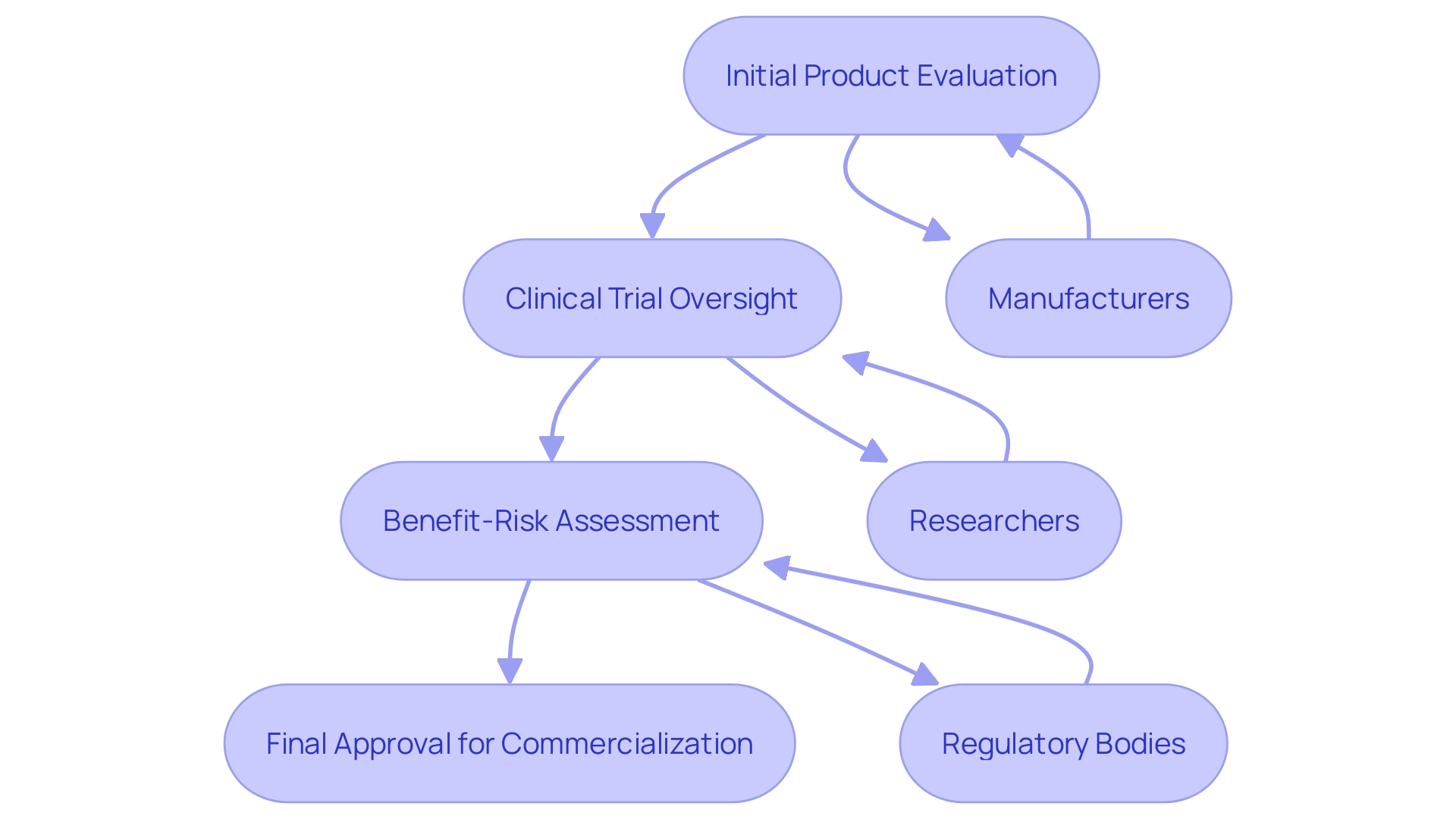
ANVISA plays a crucial role in overseeing medical equipment in Brazil, ensuring they meet rigorous safety and efficacy standards prior to entering the market. This regulatory body meticulously evaluates and approves products, providing a structured pathway for manufacturers and researchers. The agency’s oversight of clinical trials is crucial, as these trials gather essential performance and safety data, pivotal for determining the suitability of a product for commercialization.
Establishing the appropriate performance standards and information for healthcare instruments is a complicated but essential endeavor. Producers must specify the intended application of the apparatus, which includes its purpose, users, patient demographic, conditions addressed, and operational principles. This alignment ensures that the apparatus’s description, indications, and functionalities are accurately represented. Regulatory bodies like ANVISA play a significant role in guiding manufacturers through this intricate process, ensuring that the necessary preclinical and clinical investigations are conducted to demonstrate safety and effectiveness.
The benefit-risk assessment is a vital component of the design and development process, significantly influencing clinical evaluation and post-market surveillance. This thorough analysis ensures that the advantages of the healthcare product outweigh its risks, maintaining its safety and efficacy throughout its lifecycle. Interacting with governing bodies can assist in defining outcome parameters, which involves a collaborative approach with clinicians, patients, and experts to ensure relevance and feasibility.
Furthermore, ANVISA’s recent initiatives, such as enhancing access to product labels and information through digital methods, emphasize its dedication to progressing health equipment regulation. These actions not only improve the oversight structure but also encourage the responsible application of healthcare instruments, ensuring patient safety and informed usage.
Clinical Trial Approval Process and Delays
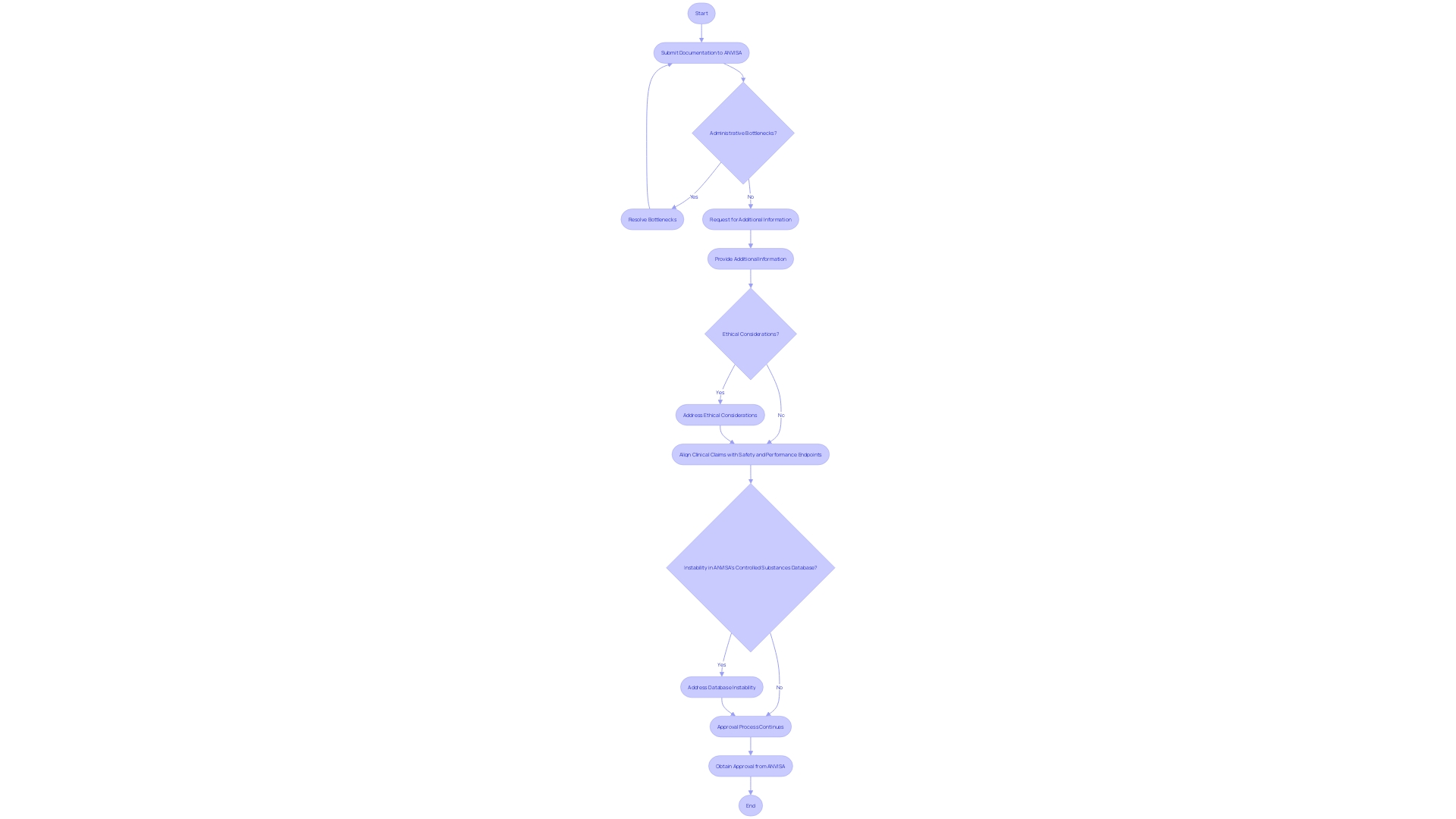
The process of obtaining approval for clinical studies involving medical devices in Brazil can be intricate and prolonged. Researchers are required to submit extensive documentation to ANVISA, which includes detailed descriptions of the study design, objectives, and methodologies. Based on recent reports, including those from GlobalData, compliance delays are often caused by administrative bottlenecks, requests for additional information, or ethical considerations. This complexity is compounded by the instability in ANVISA's controlled substances database since 2021, which has further slowed the approval process. Comprehending these possible setbacks is crucial for developers organizing tests. Recently, the EU MDR and MDCG emphasized the importance of aligning clinical claims with rigorous safety and performance endpoints, highlighting the need for precise and well-defined clinical objectives. As evidenced by the FDA's investigational equipment exemption (IDE) for the AlucentNVS study, which seeks to enhance blood circulation in haemodialysis patients, regulatory procedures can greatly influence the schedule and success of clinical studies. Consequently, developers need to be well-equipped to manage these challenges to guarantee prompt and efficient execution of tests.
Challenges in Importing Medical Devices for Clinical Trials
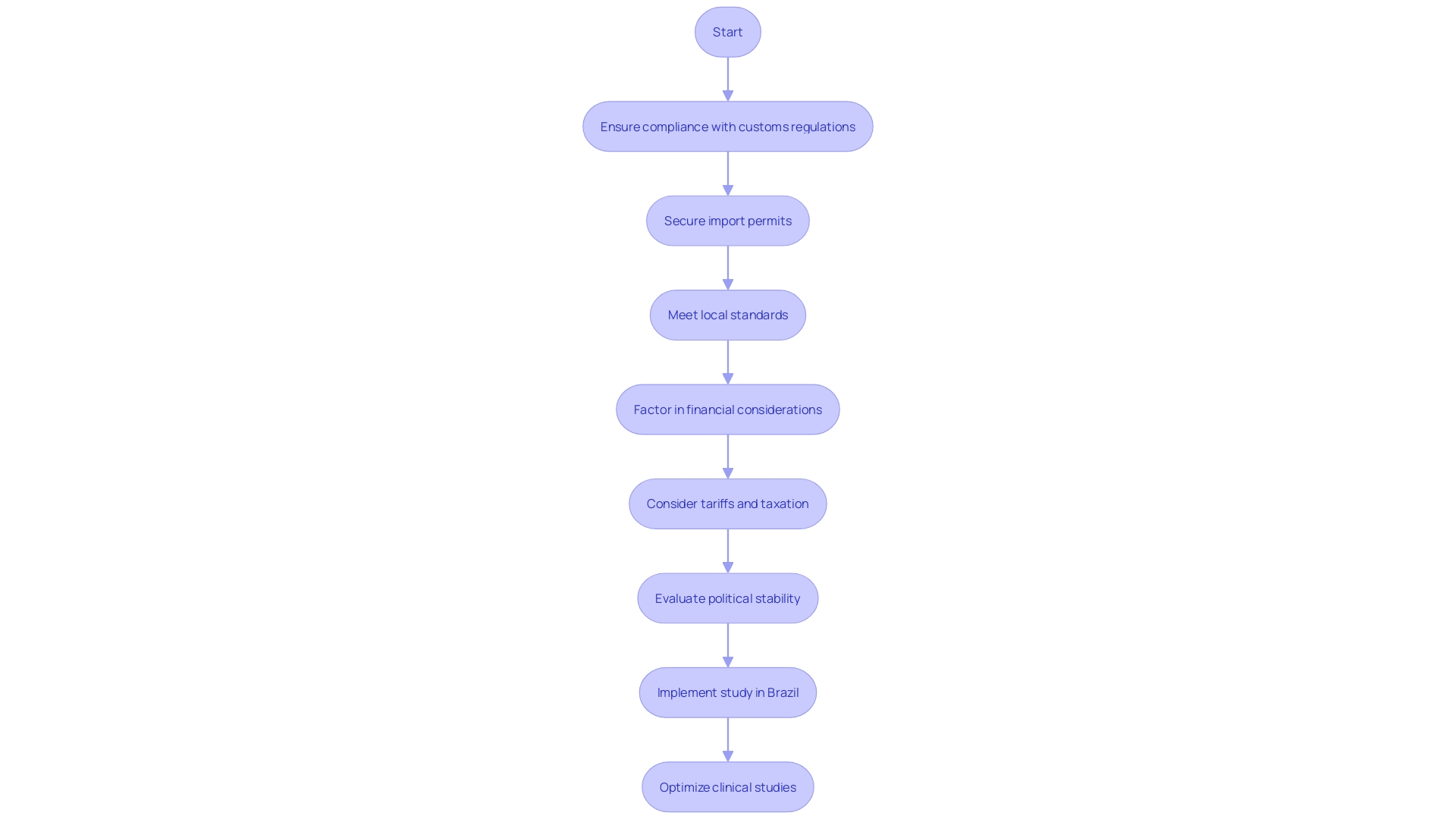
Bringing in medical equipment for clinical studies in Brazil poses various obstacles that need to be thoughtfully managed to guarantee successful study implementation. Compliance with stringent customs regulations is critical, as is securing the necessary import permits. Ensuring that all devices meet local standards is another essential step, often requiring meticulous documentation and adherence to specific guidelines.
Furthermore, possible tariffs and taxation can greatly influence the expense and practicality of carrying out experiments in Brazil. These financial considerations must be factored into the overall budget and planning process. The nation's import regulations and local political turmoil can also influence product accessibility, adding another level of difficulty to the logistics of clinical studies.
For instance, during the Russia-Ukraine conflict, multiple clinical studies were abandoned due to political instability, highlighting the importance of stable operating environments. Similarly, the ongoing Israel-Hamas conflict has caused disruptions, with several global pharma companies halting their operations in Israel.
Optimizing clinical studies in Brazil, therefore, requires a multifaceted approach. Leveraging existing public health resources, such as the extensive use of electronic medical records by over 90% of Brazil’s 57,000 primary care clinics, can help streamline the process. These records are standardized and sent to the federal government, allowing for scalable solutions that utilize already collected data.
Despite the challenges, establishing clinical studies in countries like Brazil can provide benefits, including higher recruitment rates and potentially greater access to a more engaged research staff of investigators. Nevertheless, these advantages must be weighed against the logistical challenges and compliance intricacies to attain successful experimental results.
Harmonization with International Regulatory Schemes
Aligning with global compliance criteria offers a major chance to improve the effectiveness of medical equipment trials in Brazil. By aligning with frameworks established by organizations such as the International Organization for Standardization (ISO) and the International Electrotechnical Commission (IEC), Brazil can streamline its regulatory processes. This harmonization not only facilitates easier market access for foreign manufacturers but also encourages domestic innovation.
A strong regulation-based approach, while ensuring safety and efficacy, can create the impression that market-penetrated solutions are uniformly applicable. However, achieving market access does not automatically guarantee the effectiveness or applicability of a product. With the increasing significance of digital health and wellness technologies, where regulations are less rigorous compared to healthcare instruments, there is a vital need for unified evaluation standards. This will prevent the market from becoming siloed between countries and support decision-making processes that take into account the needs of technology companies and citizens.
For example, the Brazilian government has been actively promoting digital health and has launched a new industrial health strategy aligned with local manufacturing concerns. This shift is part of a broader effort to regain leadership in global health, emphasizing the importance of multilateral institutions such as the World Health Organization. However, Brazil faces significant domestic challenges, including an overburdened healthcare system and ongoing misinformation fueled by the COVID-19 pandemic. Harmonization with international standards can help address these challenges by ensuring that digital solutions and mobile apps in the social, health, and welfare sectors are evaluated with uniform criteria, thus supporting high-quality innovations without stifling research.
Moreover, the need for harmonization is echoed in the global health landscape, as highlighted by the G20 health working group's priorities, which include digital health. The federal government's efforts to internationalize domestic concerns through the G20 framework underscore the importance of a unified approach to regulatory standards and assessment criteria. Such an approach will not only enhance the quality and availability of medical devices but also ensure that innovations meet the highest safety and performance standards, ultimately benefiting patients and healthcare providers alike.
Conclusion
The regulatory landscape for medical devices in Brazil, overseen by ANVISA, is a complex framework designed to ensure safety and efficacy while fostering innovation. This framework, influenced by both national legislation and international standards, emphasizes the importance of rigorous evaluation processes, including clinical trials and post-market surveillance, to maintain high safety standards. ANVISA plays a critical role not only in regulation but also in promoting advancements in medical technology, ensuring that patients have access to effective devices.
The approval process for clinical trials in Brazil presents challenges, including administrative delays and the need for comprehensive documentation. These hurdles can impact the timely execution of trials, necessitating careful navigation by developers. Furthermore, the importation of medical devices for clinical trials is complicated by strict customs regulations and potential tariffs, which can affect the overall feasibility and cost of conducting trials.
Despite these challenges, Brazil offers unique advantages such as higher recruitment rates, making it a valuable location for clinical research.
Harmonization with international regulatory frameworks offers a pathway to streamline processes and enhance market access for both domestic and foreign manufacturers. By aligning with global standards, Brazil can improve the efficiency of its regulatory environment and support the development of innovative medical technologies. This alignment is essential not only for regulatory consistency but also for ensuring that innovations meet the highest safety and performance standards.
Ultimately, a collaborative approach that engages all stakeholders will be crucial in advancing Brazil's MedTech sector, benefiting both patients and healthcare providers.




