Introduction
In Chile, the regulatory framework governing clinical trials for medical devices is both comprehensive and stringent, ensuring adherence to national and international standards. Overseen by the Chilean Ministry of Health, this framework mandates pre-market approval, compliance with Good Clinical Practice (GCP), and adherence to specific guidelines. Such regulations safeguard the rights and welfare of trial participants while fostering the development of innovative medical technologies.
Integral to this process is the alignment with global standards, such as those set by the European Databank on Medical Devices (Eudamed), which requires detailed safety and performance evaluations before market entry. Additionally, the incorporation of risk-benefit assessments and the submission of a Declaration of Conformity highlight Chile's commitment to maintaining high standards of medical device safety and efficacy. This regulatory environment not only protects participants but also promotes advancements in medical technology that can significantly enhance patient outcomes.
Regulatory Framework for Medical Device Clinical Trials in Chile
Chile has created an extensive set of regulations to manage the research studies of health instruments, supervised by the Chilean Ministry of Health. This framework guarantees that all clinical studies adhere to both national and international standards, safeguarding the rights and welfare of participants while fostering the advancement of innovative healthcare technologies. Important regulations encompass the requirement for pre-market authorization, following Good Clinical Practice (GCP), and adherence to particular guidelines specified for clinical trials.
The regulatory framework in Chile is designed to facilitate the safe and effective introduction of new medical products. This includes essential pre-market approval procedures to assess the safety and performance of products before they enter the market. Moreover, adherence to Good Clinical Practice (GCP) is necessary to uphold high ethical and scientific standards in research trials.
Chile's approach also aligns with international standards, such as the European Databank on Medical Equipment (Eudamed), which integrates electronic systems to collate and process information about healthcare products. Manufacturers are required to submit a Summary of Safety and Clinical Performance (SSCP) and post-market reports to address any safety issues raised through surveillance systems.
Furthermore, the regulatory structure requires a comprehensive risk-benefit evaluation, encompassing negative occurrences and possible safety issues, to guarantee that healthcare instruments fulfill their intended clinical objectives. Once these evaluations are complete, manufacturers must submit a Declaration of Conformity, certifying that the equipment complies with the Medical Device Regulation (MDR) stipulations.
This robust regulatory environment not only safeguards participants but also fosters the advancement of healthcare technologies that can significantly enhance patient outcomes.

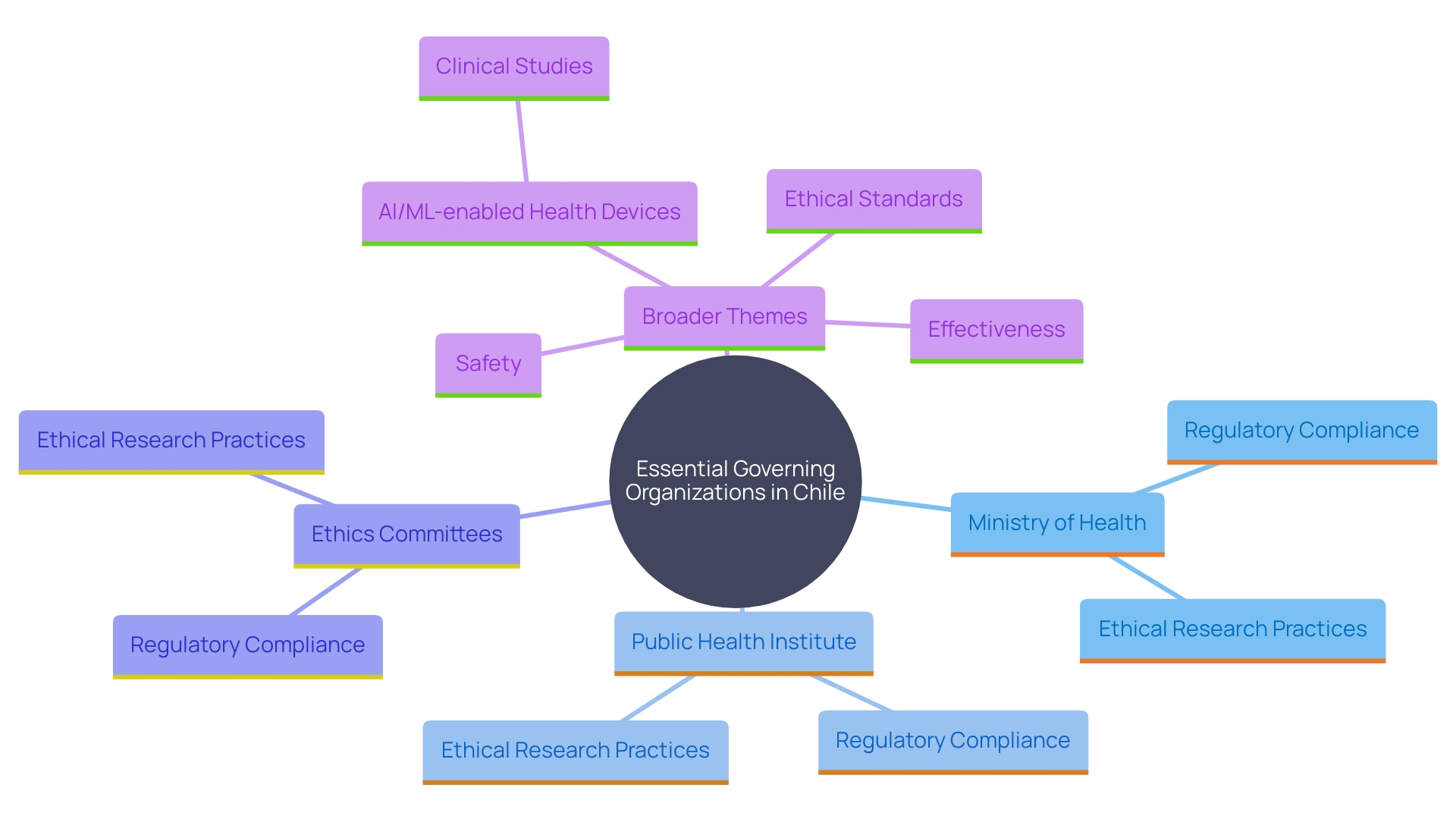
Key Regulatory Bodies and Their Roles
Numerous essential governing organizations in Chile have crucial functions in clinical studies involving healthcare equipment. The Chilean Ministry of Health functions as the primary authority, supervising the approval and monitoring of studies, ensuring adherence to national regulations. The Public Health Institute (Instituto de Salud Publica, ISP) is responsible for the assessment and registration of healthcare products, ensuring that they comply with rigorous safety and effectiveness criteria. Moreover, Ethics Committees are essential in evaluating research protocols to guarantee that ethical considerations are maintained throughout the study process, protecting participants' rights and well-being.
The scenery of health research in Chile reflects worldwide patterns, with a notable increase in the quantity of AI/ML-enabled health devices being assessed. This increase reflects the broader push for innovation in medical technology, emphasizing the need for robust regulatory frameworks to manage these advancements. Based on recent data, there is an increasing need for international trials to guarantee that new technologies function effectively across varied populations, emphasizing the significance of inclusivity in research involving health.
Chile's regulatory framework aligns with international standards, promoting the generation of dependable research data. 'This data is vital for proving the safety and effectiveness of healthcare devices, a cornerstone for market approval.'. As noted by experts, the harmonization of human subject protection regulations with international guidelines ensures that research involving humans remains efficient while protecting participants' rights. Such measures are essential as they facilitate health-related advances without compromising ethical standards.
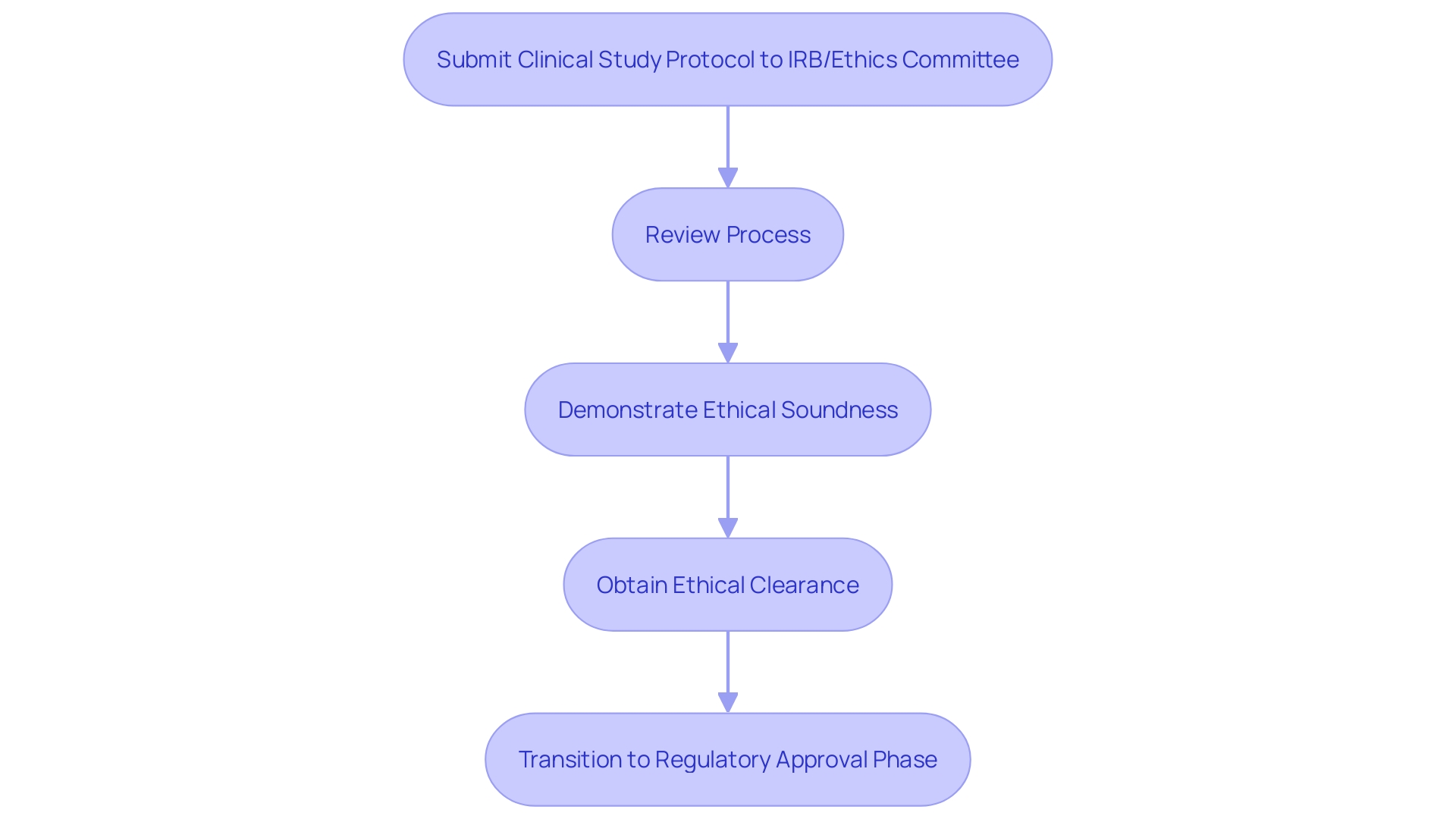
Ethical Approval and Clinical Trial Protocol Requirements
Obtaining ethical authorization is a crucial stage in the research process for medical devices in Chile. Researchers are required to submit a comprehensive clinical study protocol to an Institutional Review Board (IRB) or Ethics Committee for review. This protocol must include detailed information on the study design, objectives, methodology, and participant recruitment strategies. It is essential that the protocol demonstrates the study's ethical soundness by minimizing risks to participants and maximizing potential benefits. The importance of this process is echoed by the FDA, which emphasizes that 'well-designed studies and reliable data inform the FDA’s decision-making about a product’s benefits and risks.' 'Only after obtaining ethical clearance can the study proceed to the regulatory approval phase, a practice that aligns with global standards and ensures the protection of participant rights.'.
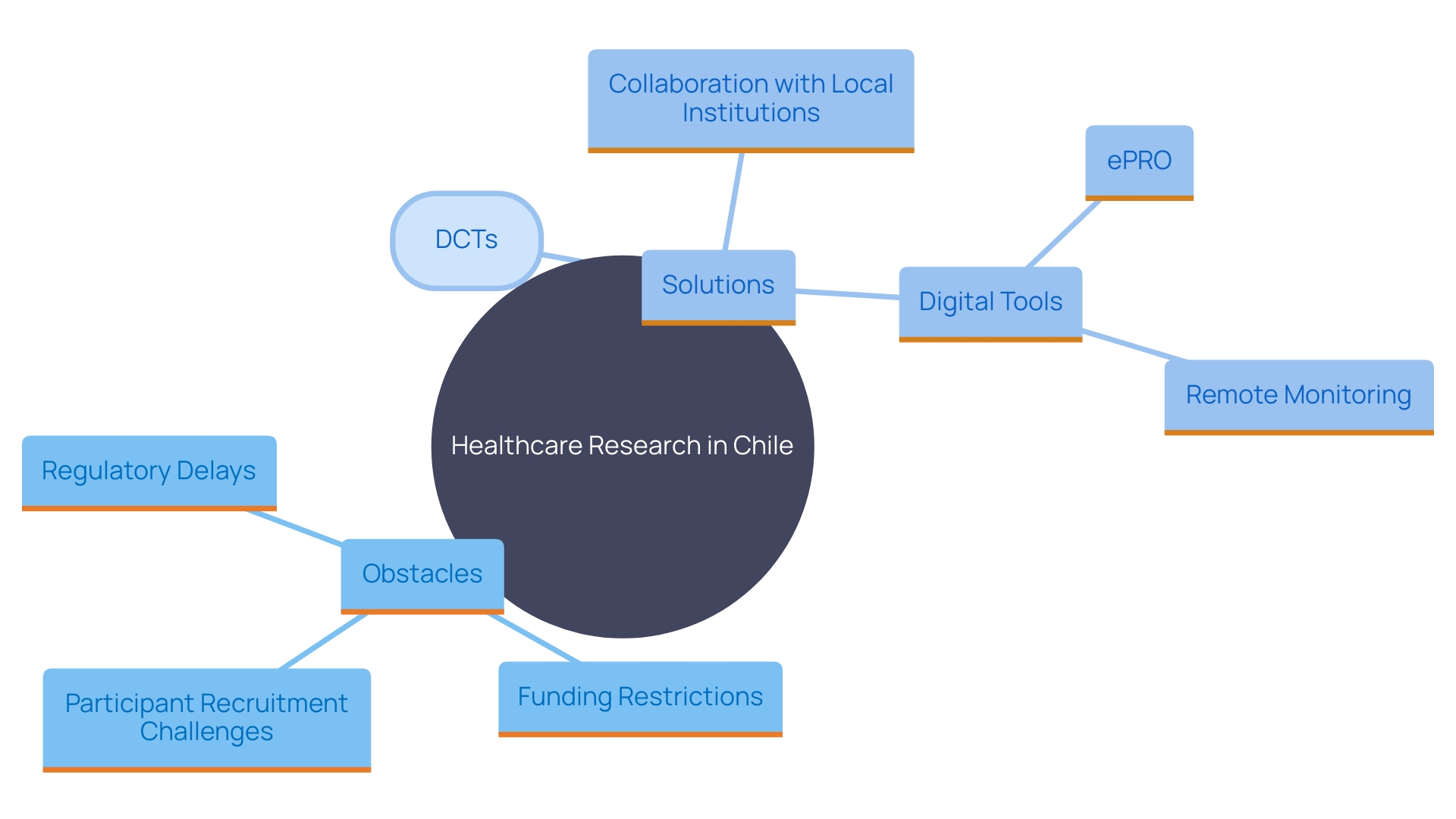
Challenges and Solutions in Conducting Medical Device Clinical Trials in Chile
Carrying out research studies for healthcare instruments in Chile encounters various obstacles, including regulatory holdups, restricted financing, and difficulties in enlisting participants. These obstacles can significantly impact the timely execution of tests and the introduction of innovative medical technologies to the market. To overcome these barriers, stakeholders should proactively engage with regulatory authorities to streamline approval processes and leverage local networks for participant recruitment. Research indicates that decentralized trials (DCTs) have become a viable solution, especially post-pandemic, with 89% of sponsors now utilizing such technologies to support at least one of their studies. By incorporating electronic patient-reported outcomes (ePRO), digital monitoring solutions, and in-home clinical services, DCTs can enhance participant retention and reduce costs. Furthermore, collaboration with local universities and research institutions can improve study feasibility and resource availability, ultimately leading to better health outcomes and increased trust in the healthcare system.
Conclusion
The regulatory framework governing clinical trials for medical devices in Chile is robust and meticulously designed to ensure safety, efficacy, and ethical compliance. The Chilean Ministry of Health, alongside other key regulatory bodies such as the Public Health Institute and Ethics Committees, plays a crucial role in overseeing the entire process. This comprehensive approach not only safeguards the rights and welfare of trial participants but also fosters innovation in medical technology through adherence to national and international standards.
Moreover, the requirement for pre-market approval, Good Clinical Practice (GCP), and thorough risk-benefit assessments reinforces Chile's commitment to maintaining high standards in clinical research. The alignment with global frameworks, such as Eudamed, ensures that the evaluation and monitoring of medical devices are both rigorous and transparent. This regulatory environment is pivotal in generating reliable clinical data, which is essential for the safety and effectiveness of new medical devices entering the market.
Despite the challenges faced in conducting clinical trials, such as regulatory delays and participant recruitment issues, solutions like decentralized clinical trials have emerged as effective strategies to enhance efficiency and inclusivity. By leveraging technology and fostering collaboration with local institutions, the clinical trial landscape in Chile can continue to evolve, ultimately leading to improved patient outcomes and advancements in healthcare. The commitment to rigorous standards and ethical practices positions Chile as a competitive player in the global medical device arena, paving the way for future innovations that can significantly enhance healthcare delivery.




