Introduction
The Special 510(k) pathway is a streamlined process designed for certain modifications to existing FDA-cleared medical devices, allowing manufacturers to implement changes more efficiently while maintaining safety and efficacy. In this article, we explore the eligibility criteria for the Special 510(k) program, the importance of evaluating performance data, the need for well-established evaluation methods, and the requirements and recommended content for Special 510(k) submissions. We also discuss examples of changes that qualify for the Special 510(k) pathway, circumstances where it may not be applicable, the review timeline, and the pros and cons of the Special 510(k) versus the traditional 510(k) submission.
Understanding the intricacies of the Special 510(k) process is crucial for medical device manufacturers in navigating the regulatory landscape and ensuring the safe and effective introduction of modified devices into the market.
What is a Special 510(k)?
The Special 510(k) pathway is intended for certain adjustments to an existing FDA-cleared medical apparatus, streamlining the process for manufacturers to implement changes. This process is less burdensome than the standard 510(k) submission, facilitating quicker modifications while maintaining safety and efficacy. As part of a Special 510(k) submission, manufacturers must provide comprehensive documentation, including a detailed description of the product, its intended patient population, and a summary of clinical testing outcomes. Labeling must cover all apparatus elements, technical specifications, and validated reprocessing instructions for reusable components.
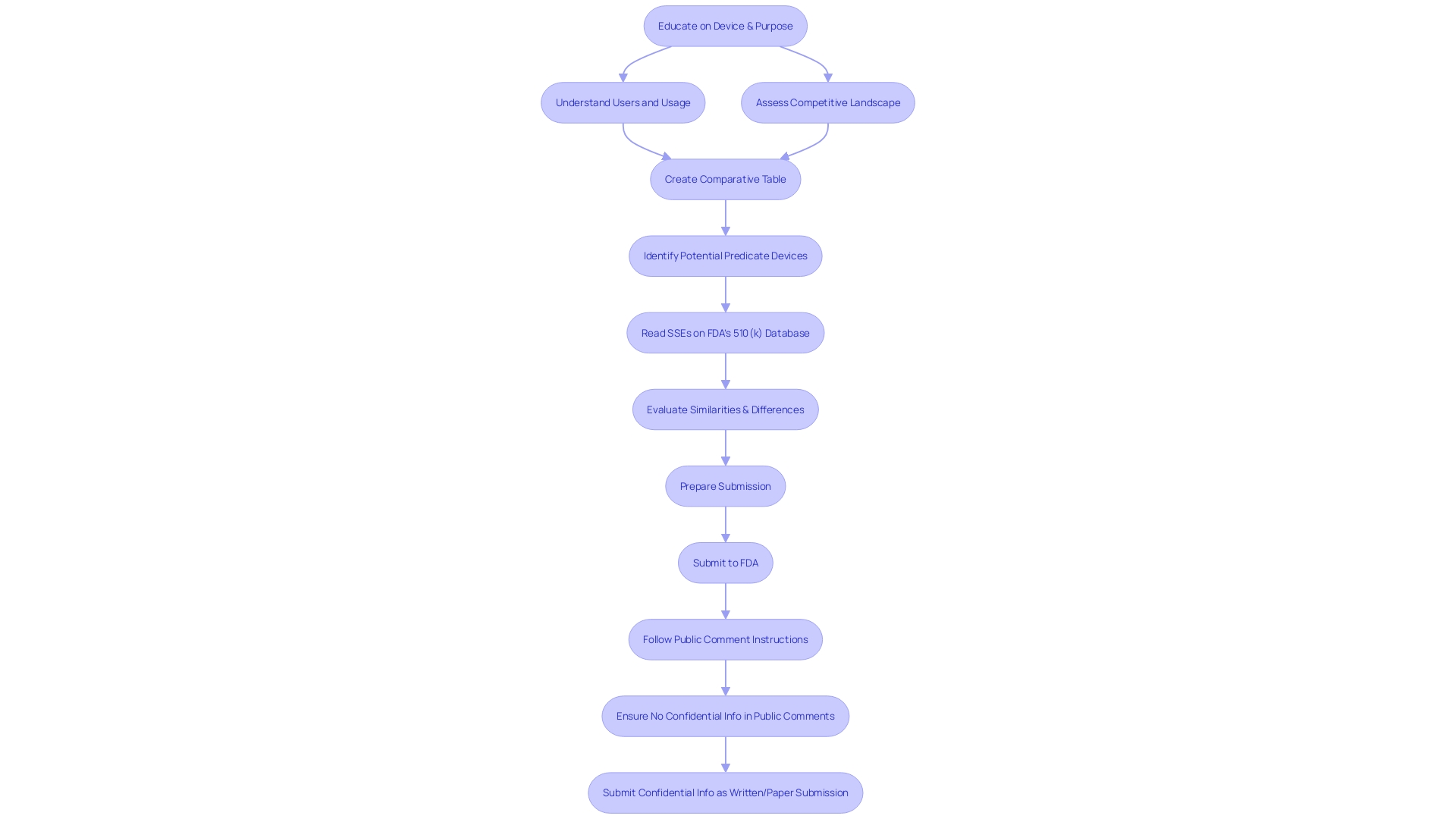
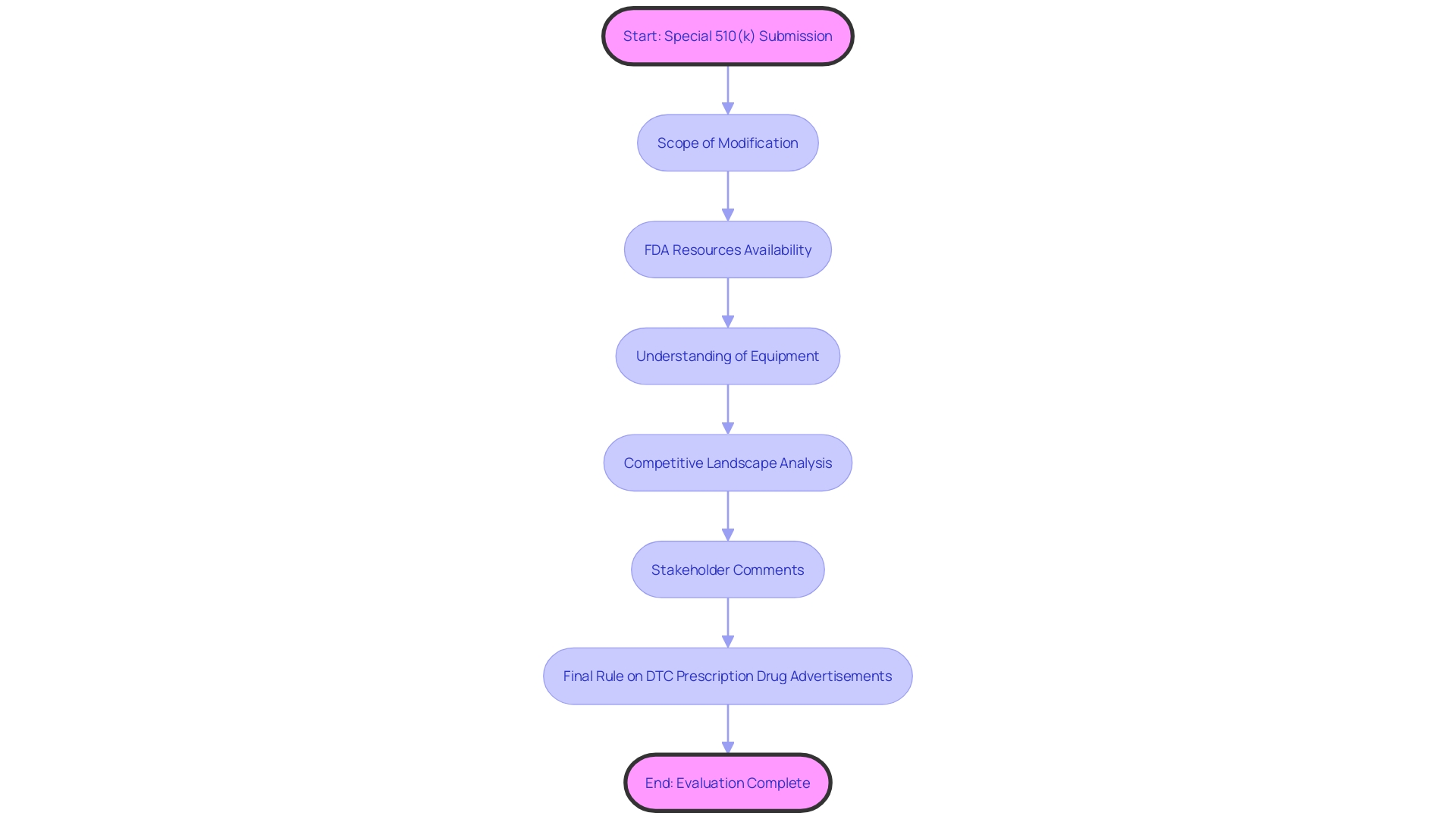
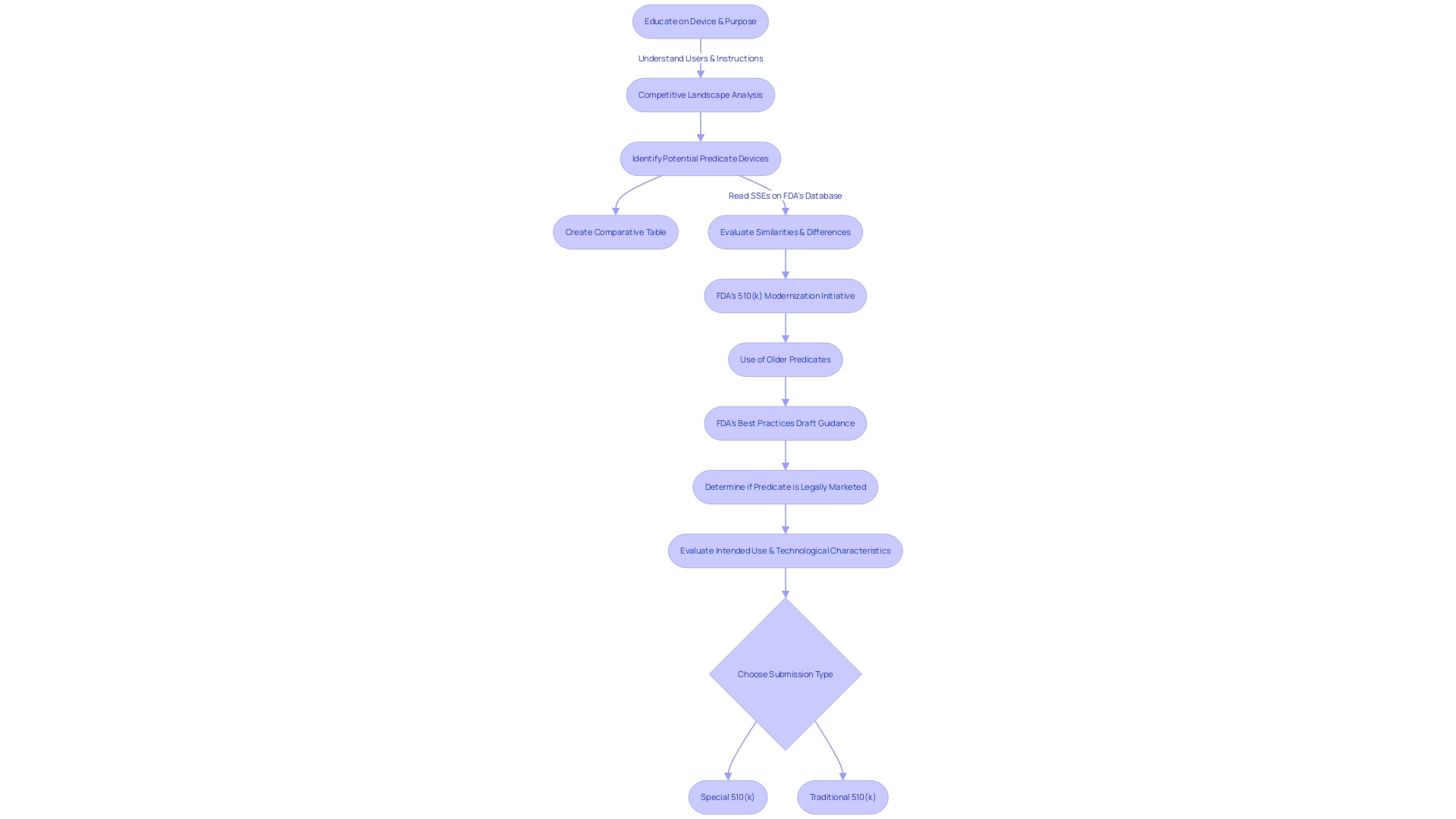
To guarantee adherence and maximize the submission, manufacturers should have a thorough understanding of the product's usage, including instructions, warnings, and cautions. A comprehensive examination of the competitive landscape is essential, often requiring cooperation with Marketing teams to identify potential precedent mechanisms that have comparable intended uses and technological characteristics. This comparative analysis should be supported by research literature, clinical studies, and FDA's Summaries of Safety and Effectiveness.
Medtronic, a global healthcare technology leader, exemplifies commitment to innovation, delivering technologies that impact lives every second. By focusing on insight-driven care and prioritizing patient experiences, Medtronic sets an industry standard for others to follow.
The FDA advocates for applicants to adhere to their guidance documents, understanding that meeting the agency's expectations for a submission is a challenge that requires careful planning and data compilation. Recent legislative and regulatory updates, as well as advisories on breakthrough programs, reflect the FDA's ongoing efforts to advance medical oversight and public health protection. The Special 510(k) program, as part of these efforts, is crucial for maintaining a balance between innovation and safety, enabling manufacturers to provide improved medical products to patients more efficiently.
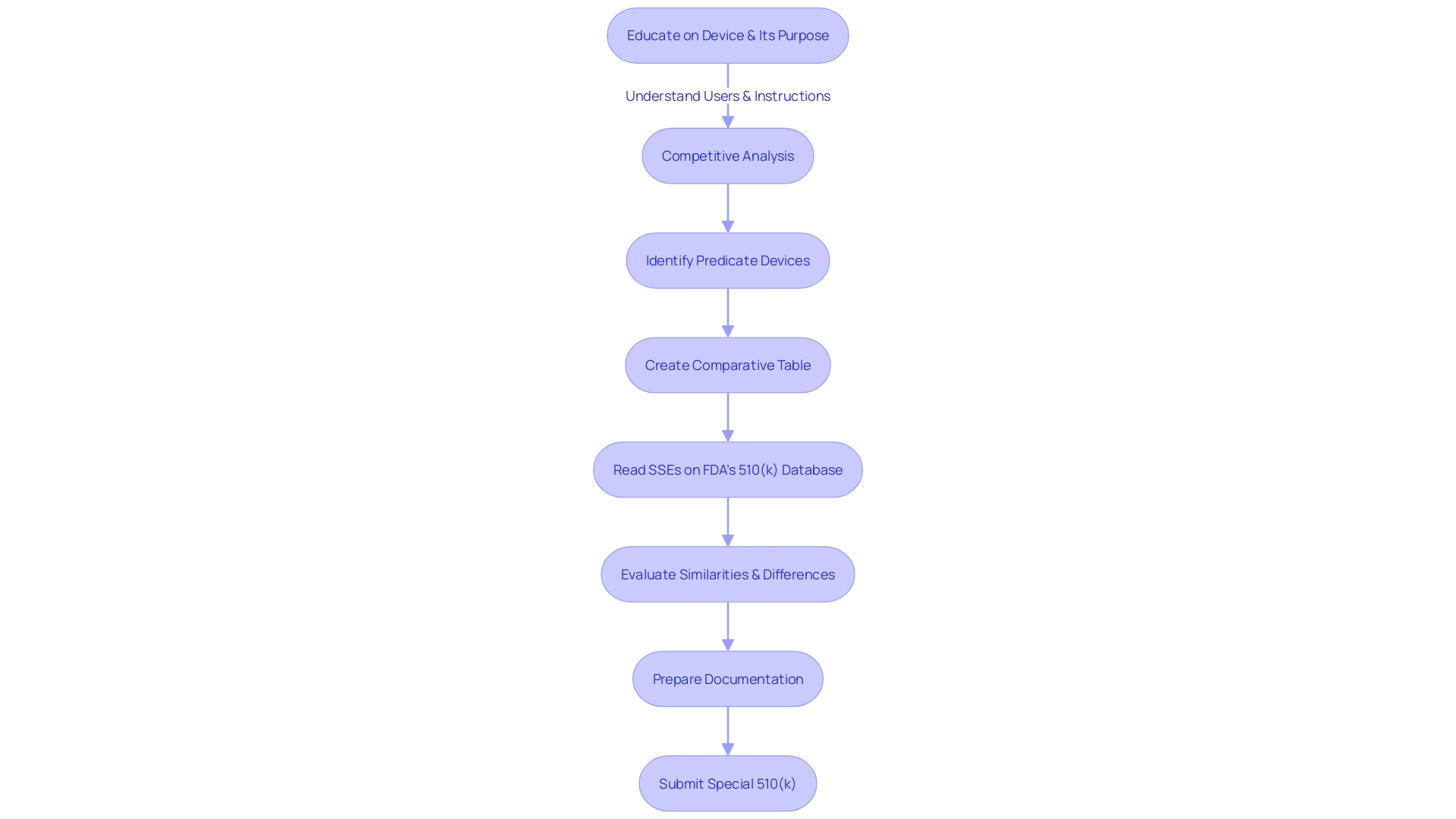
Eligibility Criteria for Special 510(k)
Producers aiming to make use of the Special 510(k) pathway for adjustments to their products must carefully evaluate whether their alterations align with the specified criteria of the program. These criteria are pivotal in confirming that the modifications are indeed suitable for the Special 510(k) process. Firstly, a thorough understanding of the instrument in question is essential, including its intended users—ranging from healthcare professionals such as clinicians, physicians, and dentists to patients—and detailed instructions for use, complete with warnings and precautions. It is also advantageous to examine the competitive landscape from a marketing perspective to identify similar products. This involves a thorough review of available content including research literature, clinical studies, and instructional material to pinpoint potential predicate objects that share the intended use and possess similar technological characteristics. Subsequently, a comparative analysis table should be constructed.
In the context of the FDA's efforts to refine the 510(k) submission process, the selection of an appropriate predicate is integral. To establish this, the product must not only be legally marketed (currently registered with the FDA) but also share the intended use with the potential predicate. Moreover, technological differences should not evoke new questions regarding safety and effectiveness. This selection process has been encapsulated in the FDA's draft guidance document, released on September 7, 2023, as a part of their initiative to modernize the 510(k) review process and establish best practices for predicate selection. The guidance underscores the importance of utilizing predicates that, while potentially older, have accrued valuable long-term safety data, thus offering distinct advantages for supporting a 510(k) submission.
The requirements for Special 510(k) eligibility are designed to uphold the FDA's dedication to protecting public health by ensuring the safety, effectiveness, and security of medical equipment. As an authoritative body responsible for regulating a wide array of products, including medical instruments, the FDA's standards are stringent and aim to prevent any compromise on public health. It is imperative that manufacturers understand the gravity of these criteria and adhere to the FDA's guidance to navigate the regulatory landscape successfully.
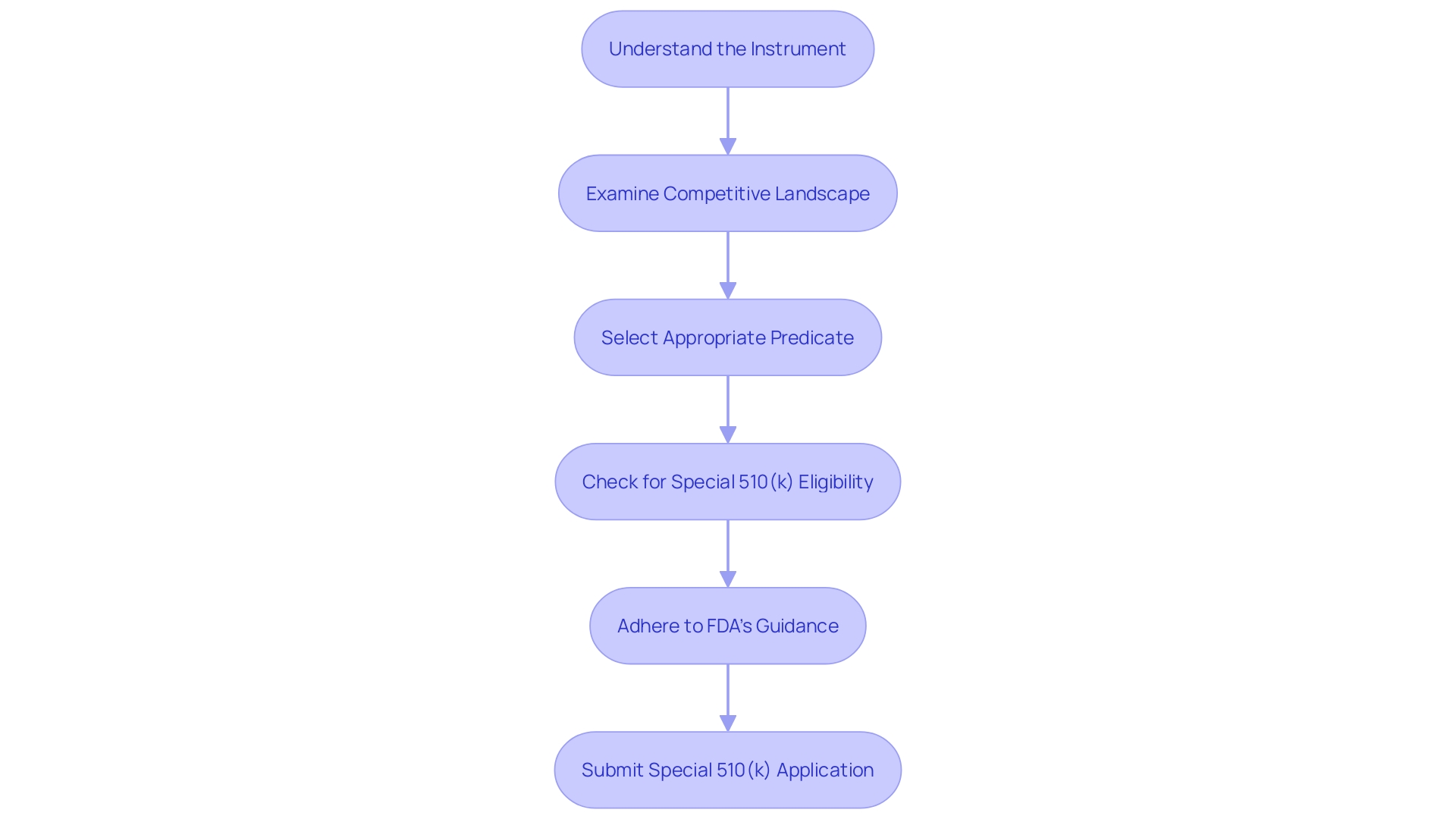
Is it a change to the manufacturer’s own device?
When evaluating improvements to medical equipment presently available, it is essential to evaluate the alterations within the framework of the initial product created and produced by the identical organization. These modifications should target enhancing the safety and effectiveness of the equipment, while adhering to the organizational and technical structure of Industry 4.0. This entails integrating cutting-edge technology stacks or supporting technologies to streamline data sources, enhancing overall equipment effectiveness, and optimizing key performance indicators specific to manufacturing. Learning from past implementations, such as the 22 detailed in the report 'Industry 4.0 check-in: 5 learnings from ongoing digital transformation initiatives,' can guide manufacturers through potential hurdles. For instance, companies like Medtronic, with a strong ethos of addressing complex health challenges, continually innovate to deliver technologies that impact lives every moment. Their method of altering the equipment, guided by a goal of reducing discomfort and prolonging life, could be used as a model for guaranteeing that equipment enhancements result in significant advantages for patients and healthcare professionals. In addition, it is important to involve potential users of the equipment, whether they are healthcare professionals or patients, to confirm that the alteration truly meets a real requirement, in line with the intended purpose and recommended uses of the apparatus.
In the regulatory arena, transparency is key. When submitting comments related to alterations, it is the duty of the manufacturer to omit sensitive data to safeguard privacy and exclusive particulars. The WEEE and RoHS Directives further emphasize the significance of sustainability in manufacturing and disposal of devices, highlighting the requirement for environmental stewardship throughout the lifecycle of the product. These guidelines and directives not only shape the development and alteration processes but also serve as a testament to the industry's commitment to innovation balanced with social responsibility and regulatory adherence. As the landscape changes, keeping up to date with legislative, regulatory, and payment policy updates, including those affecting the Breakthrough Devices Program, is crucial for manufacturers aiming to excel in the medical equipment sector.
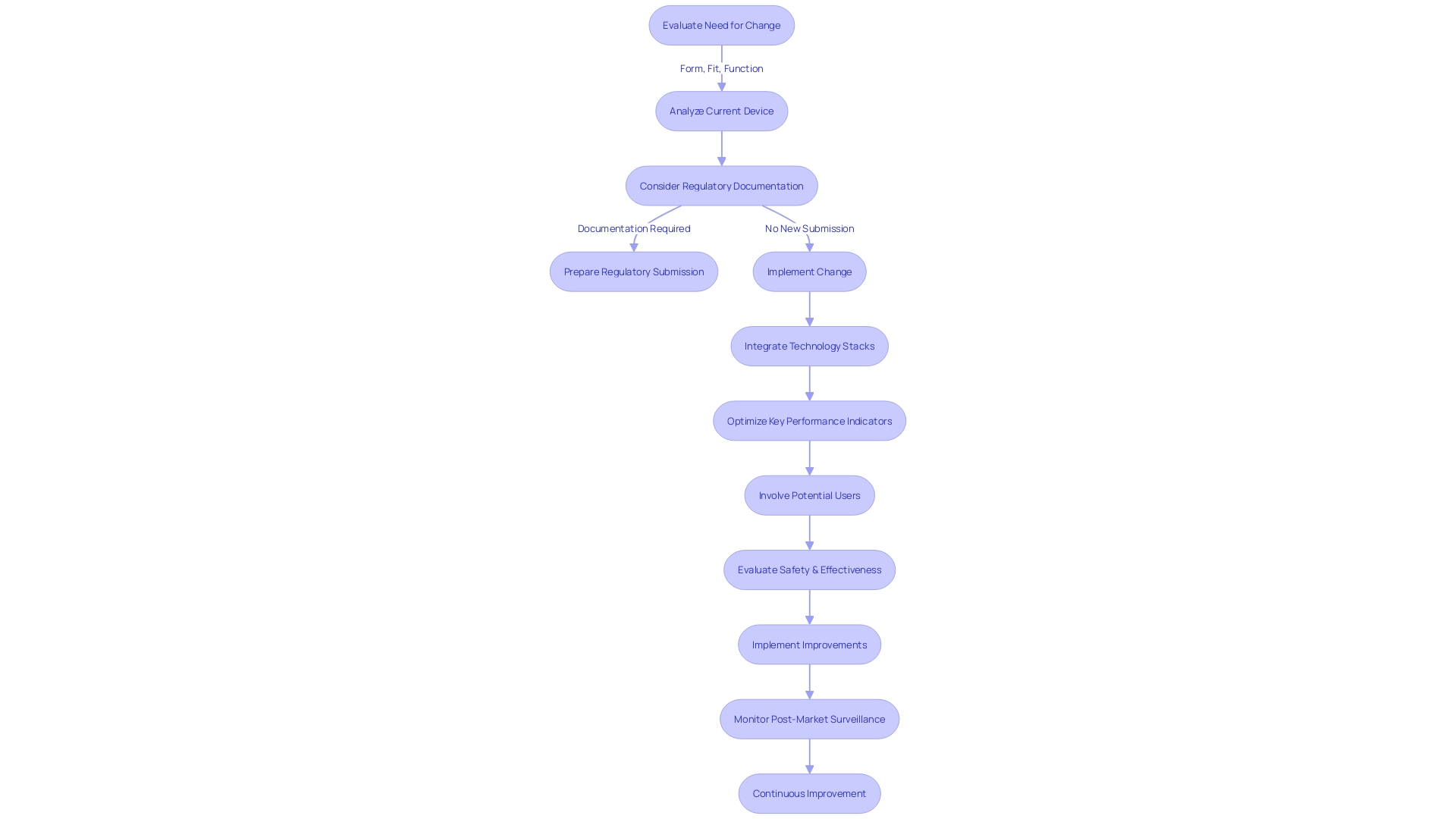
Are performance data needed to evaluate the change?
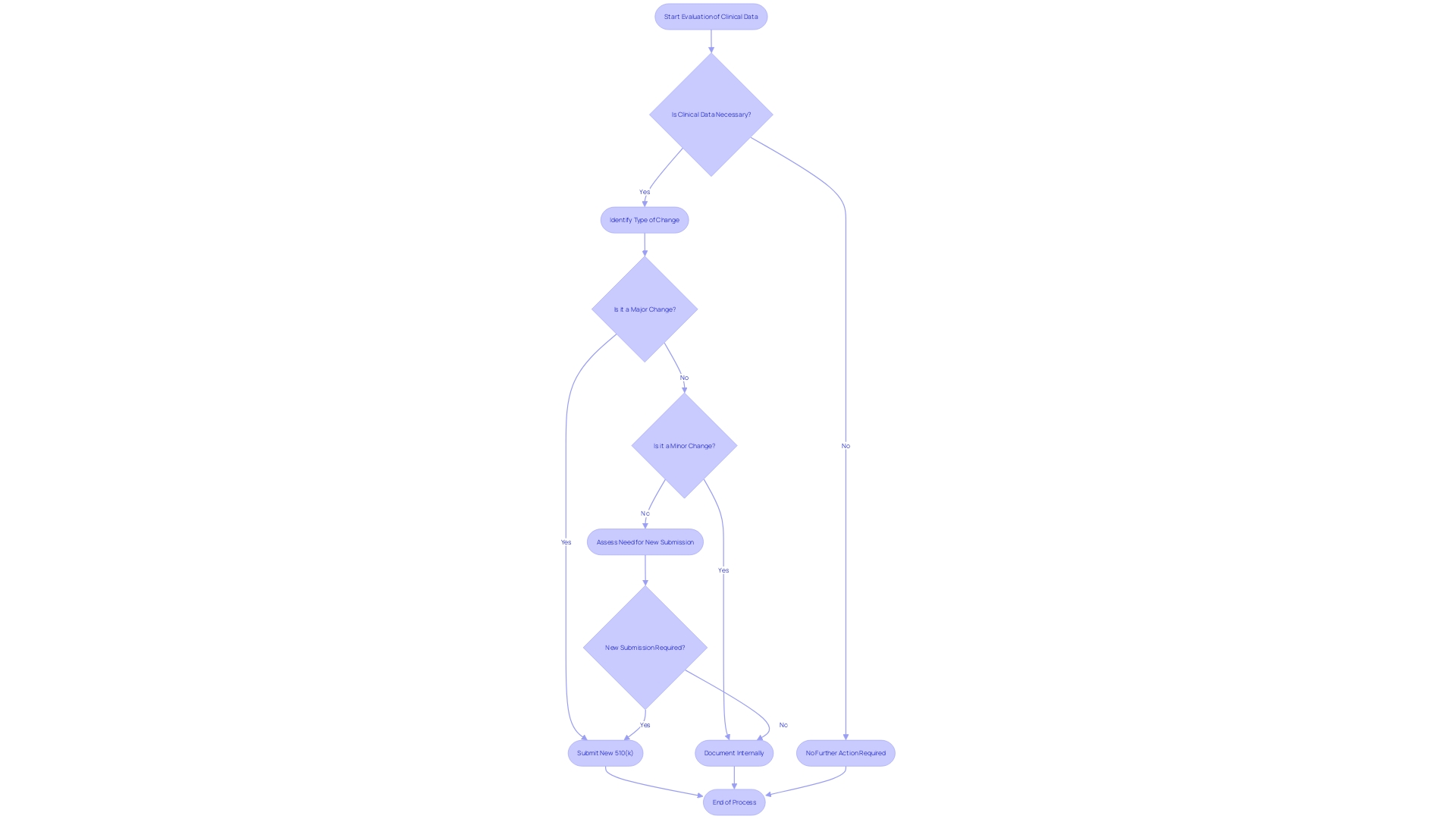
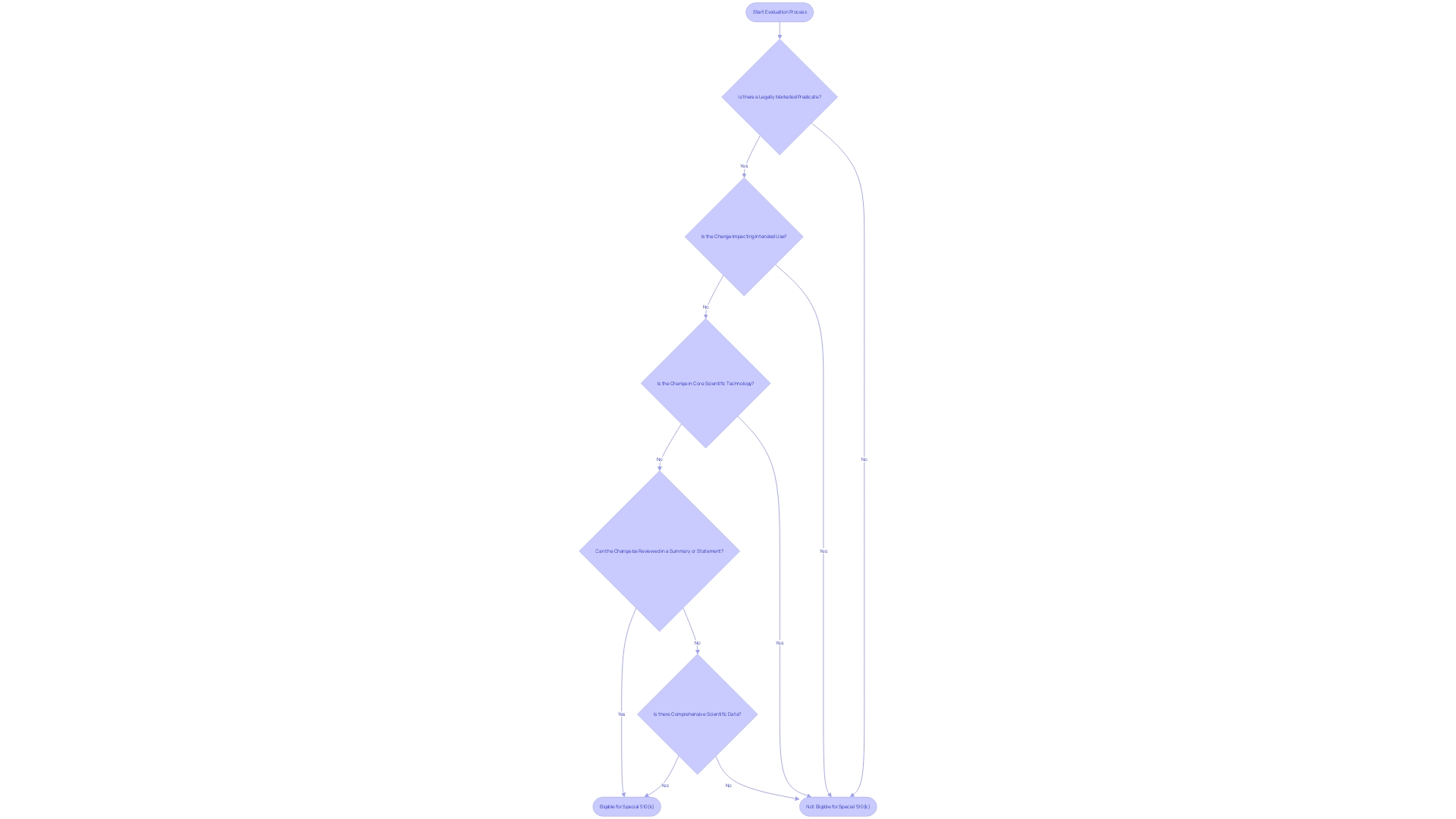
When a medical apparatus goes through an alteration that could greatly impact its safety or efficacy, the FDA may demand the submission of supplementary performance data to assess the influence of the change. This performance data could encompass a range of evidence from clinical studies to non-clinical laboratory testing, depending on the nature of the change and the potential risks involved. For example, clinical data may be necessary when there is a change in the intended use of the equipment, a new indication for use, or when modifications are made to the instrument that could affect clinical outcomes. It is important to mention that in such cases, submission via the Special 510(k) pathway may not be suitable, as this pathway is reserved for changes that do not significantly affect the equipment's safety and effectiveness.
Recent FDA observations have highlighted concerns over the integrity of data submitted by third-party test labs. With a rise in untrustworthy data, such as falsified or replicated information, the FDA's capacity to approve marketing authorization is compromised, which can hinder the accessibility of new medical instruments to patients and healthcare providers. To address these concerns, the FDA underscores the importance of precise, dependable, and sturdy clinical data, particularly for products that pose a greater potential risk. Clear guidance is provided by the FDA, outlining when clinical data is essential for 510(k) submissions, ensuring that products meet the required standards for safety and effectiveness.
In light of these considerations, it is crucial for manufacturers and sponsors to rigorously evaluate their data and ensure its validity. The FDA's draft guidance offers a clear framework for identifying scenarios where clinical data is needed, thereby assisting in the preparation of 510(k) submissions. By following these guidelines and upholding strict data standards, manufacturers can contribute to the secure and efficient introduction of medical equipment into the market.
Is there a well-established method to evaluate the change?
To be eligible for a Special 510(k) submission, the change in question must be evaluable through a recognized and established method. This criterion is met when there is clear guidance from the FDA or consensus standards that are widely acknowledged and referenced to assess the changes. This procedure guarantees that changes to healthcare equipment persistently fulfill the strict criteria of safety and efficiency, strengthening the FDA's dedication to safeguarding public health, as echoed in the agency's recent release of guidelines for direct-to-consumer prescription drug advertisements. The updated guidelines emphasize the importance of presenting information in an easily understandable manner for consumers, which is also a critical aspect of the Special 510(k) pathway. Submissions should therefore be comprehensive and articulate, providing a transparent synopsis of the change supported by scientific evaluation. By following such standards, medical product manufacturers can uphold compliance and contribute to the safety and security of medical items.
Can the data be reviewed under a summary or risk-analysis format?
To accelerate the review process of changes to medical equipment, the Special 510(k) Program allows manufacturers to present a brief summary or risk analysis to the FDA. This method enables the FDA to evaluate the modifications efficiently, concentrating on the impact of the alteration instead of re-evaluating the entire equipment. For instance, when reclassifying ultrasound cyclodestructive equipment from class III to class II, it was emphasized that the alteration in regulatory controls could enhance patient access without increasing the occurrence of medical issues. The submission under this program should include a detailed description of the patient population, instructions for use with warnings and precautions, and a comprehensive summary of clinical testing outcomes, including any adverse events. Moreover, it should clarify the operation of the apparatus, treatment process, and component particulars, guaranteeing that all technical specifications are clearly outlined. As the FDA continually updates standards for clarity and transparency in advertising and labeling, submissions must also adhere to these evolving guidelines to maintain public trust and ensure safety.
Special 510(k) Submission Requirements
As part of the FDA's commitment to safeguard public health, the agency stipulates meticulous requirements for Special 510(k) submissions. These are created to encapsulate all the vital details the FDA needs to evaluate any modifications made to a medical apparatus. The submission must showcase a comprehensive understanding of the apparatus, its users, usage instructions, and safety precautions. Furthermore, it is crucial to possess a thorough understanding of the competitive environment, including a comparative examination of potential precursor devices. Recognizing the fast development of the industry, the FDA's regulations strive to strike a balance between the necessity for comprehensive evaluation and the distinct technical and operational characteristics of the medical instrument sector, as well as the requirement for conformity without impeding innovation. The FDA's recently finalized rule on direct-to-consumer prescription drug advertisements underscores the agency's dedication to clear and understandable communication, a principle that is also reflected in the requirements for Special 510(k) submissions.
Recommended Content for Special 510(k) Submission
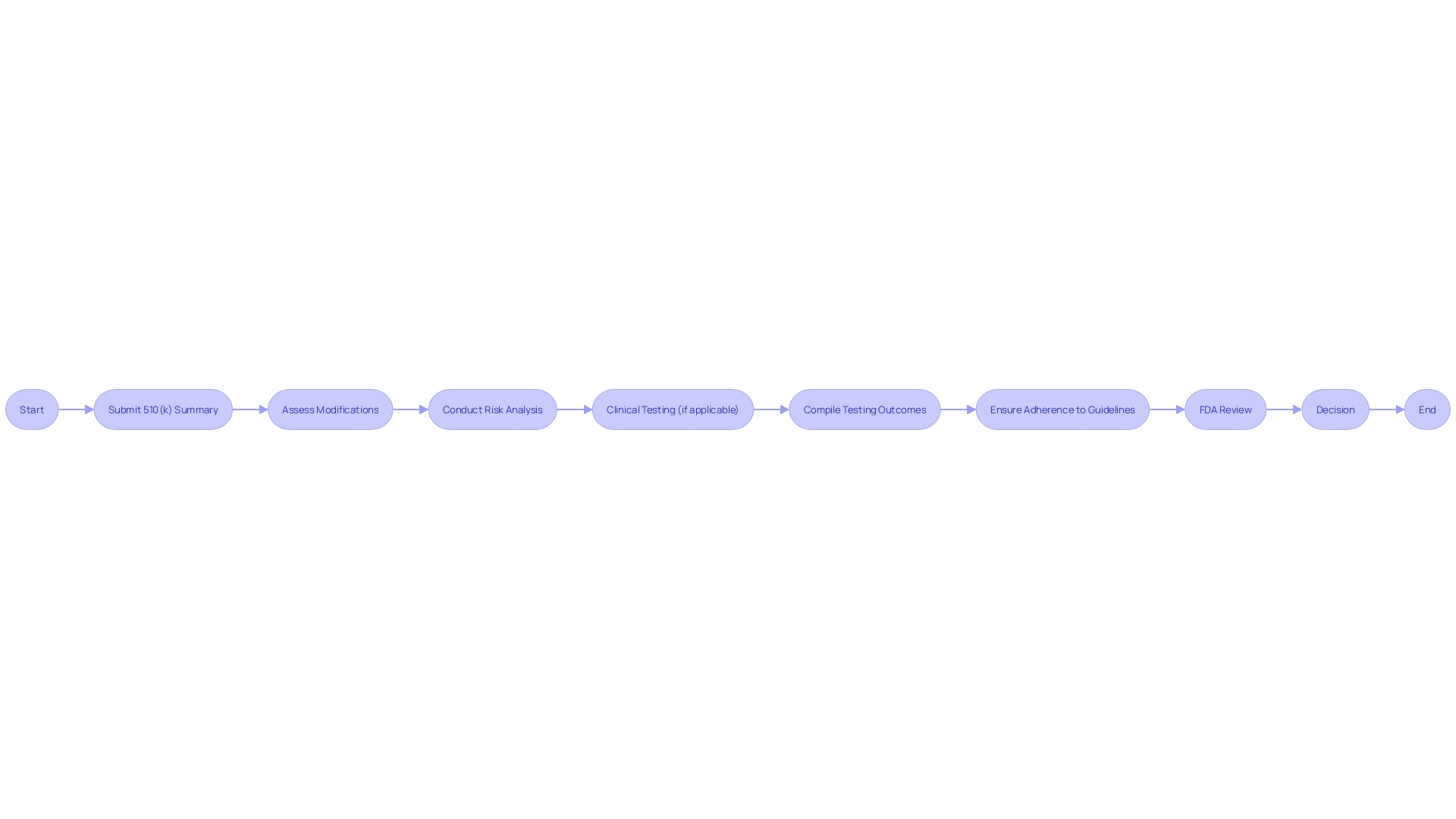
When submitting a Special 510(k), it's crucial to specify modifications and their ramifications on the equipment's safety and effectiveness. A well-prepared submission should include a comprehensive comparative analysis, clearly outlining the differences and similarities between the modified object and its predicate. Furthermore, it's crucial to explain any alterations in the performance specifications of the equipment and how these may impact clinical outcomes. The FDA requests comprehensive documentation of the alteration, including corroborating evidence from non-clinical or clinical data, ensuring the modified equipment remains as safe and effective as the original. Inclusion of relevant standards or guidelines followed, alongside a thorough risk analysis, can further strengthen the submission. The aim is to give the FDA enough information to evaluate whether the product, as altered, still conforms to the essential regulatory criteria for market entry.
Examples of Changes and Steps for Determining Eligibility
Navigating the intricacies of a Special 510(k) submission can be complex, but understanding the types of changes that qualify can aid in a smoother approval process. A Special 510(k) may be suitable when modifications are made to an apparatus that was already cleared under a traditional 510(k), and these changes do not affect the intended use or alter the fundamental scientific technology of the apparatus. Examples of changes that might be eligible for a Special 510(k) include minor design alterations or software updates that do not introduce new risks.
The FDA's draft guidance titled 'Recommendations for the Use of Clinical Data in Premarket Notification [510(k)] Submissions' delineates specific scenarios where clinical data is pivotal alongside non-clinical data to establish substantial equivalence. These scenarios cover a spectrum of situations, including differences in indications for use between the new tool and the reference, technological variations that could impact safety and effectiveness, cases where substantial equivalence cannot be ascertained through non-clinical testing alone, and situations where new or heightened risks identified in the reference tool could necessitate clinical data for the new tool.
These guidelines highlight the importance of a comprehensive evaluation method, integrating both clinical and non-clinical testing, to ensure the safety and effectiveness of medical instruments. The given instances in the preliminary advice act as important points of reference for interested parties in deciding the timing and kind of information needed in a 510(k) application, ensuring that the alterations made to the equipment align with FDA criteria and uphold the utmost level of patient well-being.
Circumstances Ineligible for Special 510(k)
Certain alterations to medical equipment may qualify for a simplified evaluation procedure under the Special 510(k) program. However, it's crucial to identify when this pathway is not applicable. For example, if the alteration impacts the intended use of the apparatus or changes its core scientific technology, a Special 510(k) may not be appropriate. Moreover, if the alteration is without a legally marketed predicate, or if the comparison to the predicate necessitates a comprehensive evaluation of scientific data to establish substantial equivalence, the Special 510(k) pathway would be unsuitable. Comprehending these particular circumstances guarantees that submissions are in line with FDA requirements and aids in preserving the integrity and safety of medical instruments on the market.
Special 510(k) Review Timeline
The evaluation period of a Special 510(k) submission is influenced by multiple parameters. These include the scope of the modification to the medical apparatus and the current demands on the FDA's resources. While the FDA strives to complete the review process within 30 days after receiving a Special 510(k) submission, it's important to recognize that each submission is unique. A thorough understanding of the equipment in question is necessary, covering knowledge of its users, such as clinicians and patients, and its instructional guidelines, which outline any warnings and precautions. Moreover, understanding the competitive landscape, which includes studying literature, clinical studies, and competitor information, is essential in identifying a suitable counterpart that has the same intended use and technological features as the subject apparatus. The FDA's dedication to public health and safety extends to a thorough assessment of medical equipment to ensure their effectiveness and security. Stakeholders are encouraged to contribute comments and insights during regulatory review periods, and these comments become part of the public record after thorough review by the Health and Human Services Department. The recent FDA final rule on direct-to-consumer prescription drug advertisements underscores the agency's dedication to clear and transparent communication, setting standards for the presentation of major side effects and contraindications in consumer-friendly language. This level of detail and public interaction is representative of the FDA's transparent regulatory process, ensuring that all voices are heard and considered in the journey to bring safe and effective medical products to market.
Pros and Cons of Special 510(k) vs Traditional 510(k)
Selecting between a Special 510(k) and a traditional 510(k) submission involves weighing the unique benefits and drawbacks of each pathway. The Special 510(k) program may provide a streamlined review process for manufacturers making minor changes to an existing product or modifications that do not alter the intended use or change the fundamental scientific technology of the item. This can lead to a faster clearance time, which is crucial in the fast-paced medical equipment industry where getting to market expeditiously can be a significant competitive advantage.
Nonetheless, it's crucial for manufacturers to grasp the intricacies of the apparatus, encompassing its usage by diverse stakeholders such as clinicians, physicians, dentists, and patients, and to be fully cognizant of the instructions, warnings, and cautions involved. A comprehensive analysis of competition, including a review of research literature, clinical studies, and competitor marketing materials, is necessary to identify suitable precedent items and create a comparative analysis table.
On the other hand, the traditional 510(k) process, while generally taking longer, allows for a broader scope of changes and may be necessary if the equipment's modifications are substantial. It's also important to take into account that some equipment cleared through the 510(k) process, including those fast-tracked, have later been associated with patient injuries, underscoring the importance of a thorough safety and effectiveness evaluation. Manufacturers must carefully examine the Summaries of Safety and Effectiveness Data (SSEDs) available in the FDA's 510(k) database to assess similarity and differences comprehensively.
Ultimately, the choice between Special and traditional 510(k) submissions will depend on the specific circumstances of the device update, and manufacturers must navigate these options with a detailed understanding of the regulatory implications and a commitment to patient safety.
Conclusion
In conclusion, the Special 510(k) pathway offers a streamlined process for medical device manufacturers to implement modifications while maintaining safety and efficacy. Manufacturers must carefully assess their changes to ensure eligibility and provide comprehensive documentation to support their submissions.
Performance data may be required to evaluate the impact of modifications, and manufacturers should rigorously evaluate their data to meet the FDA's standards. It is important to use recognized and established evaluation methods, as outlined by the FDA or consensus standards.
When submitting a Special 510(k), manufacturers should provide detailed documentation of the modifications, including a comparative analysis and changes in performance specifications. The goal is to provide the FDA with sufficient information to assess compliance with regulatory requirements.
While the Special 510(k) pathway offers a faster review process for minor modifications, manufacturers should consider the specific circumstances of their device updates. The choice between the Special and traditional 510(k) pathways depends on factors such as the extent of the modifications and the need for a broader scope of changes.
Overall, understanding the intricacies of the Special 510(k) process is crucial for manufacturers to navigate the regulatory landscape and ensure the safe and effective introduction of modified devices into the market. By adhering to the eligibility criteria, providing comprehensive documentation, and evaluating performance data, manufacturers can contribute to the advancement of medical device oversight and public health protection.




