Overview
This article delineates the essential steps involved in designing device trials, underscoring the significance of structured methodologies throughout various phases of clinical studies. It articulates the critical roles of:
- Safety assessments
- Regulatory compliance
- Effective recruitment strategies
Illustrating how these elements collectively ensure the successful evaluation of medical devices in real-world conditions.
Introduction
In the intricate world of medical research, clinical trials are the cornerstone for ensuring the safety and efficacy of new medical interventions. These systematic investigations not only pave the way for innovative drugs and devices but also adhere to rigorous methodologies that span multiple phases, each with its own set of objectives. As the landscape evolves, understanding the nuances of clinical trials becomes essential for researchers, developers, and stakeholders alike.
From the pivotal role of randomization and control groups to the challenges faced in medical device trials, this article delves into the critical components that shape successful clinical investigations. Furthermore, it highlights emerging trends and innovations that promise to redefine the future of clinical research, ensuring that patient safety and effective treatment remain at the forefront of medical advancements.
Understanding Clinical Trials: Key Concepts and Definitions
Clinical studies are systematic investigations designed to assess the safety and efficacy of medical interventions, which encompass both drugs and medical devices. These studies are organized into distinct phases, each tailored to achieve specific objectives and employing customized methodologies. A key distinction exists between interventional and observational studies: interventional studies actively assign participants to specific treatments, whereas observational studies monitor outcomes without direct intervention.
The significance of randomization in experimental studies is paramount. Randomization mitigates bias, ensuring that observed treatment effects stem from the intervention rather than confounding variables. Recent statistics indicate that in 2022, approximately:
- 106 early phase-I interventional studies
- 2,478 phase-I interventional studies
- 4,219 phase-II interventional studies
- 2,031 phase-III interventional studies
- 585 phase-IV interventional studies
were active across various health conditions, underscoring the extensive application of these methodologies in research.
Furthermore, the role of control groups is critical in establishing a benchmark against which the efficacy of interventions can be evaluated. This approach is essential for validating the results of medical studies, particularly concerning medical devices, where safety and effectiveness are crucial. Companies like bioaccess® provide comprehensive management services for studies, including:
- feasibility assessments
- site selection
- compliance evaluations
- setup
- import permits
- project oversight
- reporting
ensuring meticulous management of each study phase.
With over 20 years of experience in Medtech, bioaccess® brings specialized expertise and adaptability to effectively navigate the complexities of research processes.
Current trends in medical studies emphasize enhancing development pathways, not only to secure regulatory approval but also to ensure commercial viability. As articulated by Max Baumann, Head of Execution, "We expect continued focus on optimizing the development journeys of assets to achieve not only an approval-enabling endpoint but to qualify for commercial success." This reflects a growing emphasis on identifying and evaluating suitable outcomes, which is crucial for the success of studies, especially in emerging areas like psychedelics, as noted by Joseph Tucker from Enveric.
A comprehensive analysis of medical studies registered from 1999 to June 2024 reveals that the USA leads in the number of studies, with a notable focus on non-communicable diseases. This data illustrates the demographic diversity in study participation, with a majority of research involving both male and female adult participants. Such insights are invaluable for understanding the landscape of medical studies and the methodologies that underpin them, providing a robust foundation for designing device trials.
With bioaccess®'s expertise in managing Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Follow-Up Studies, clients can confidently navigate the complexities of clinical research in Latin America.
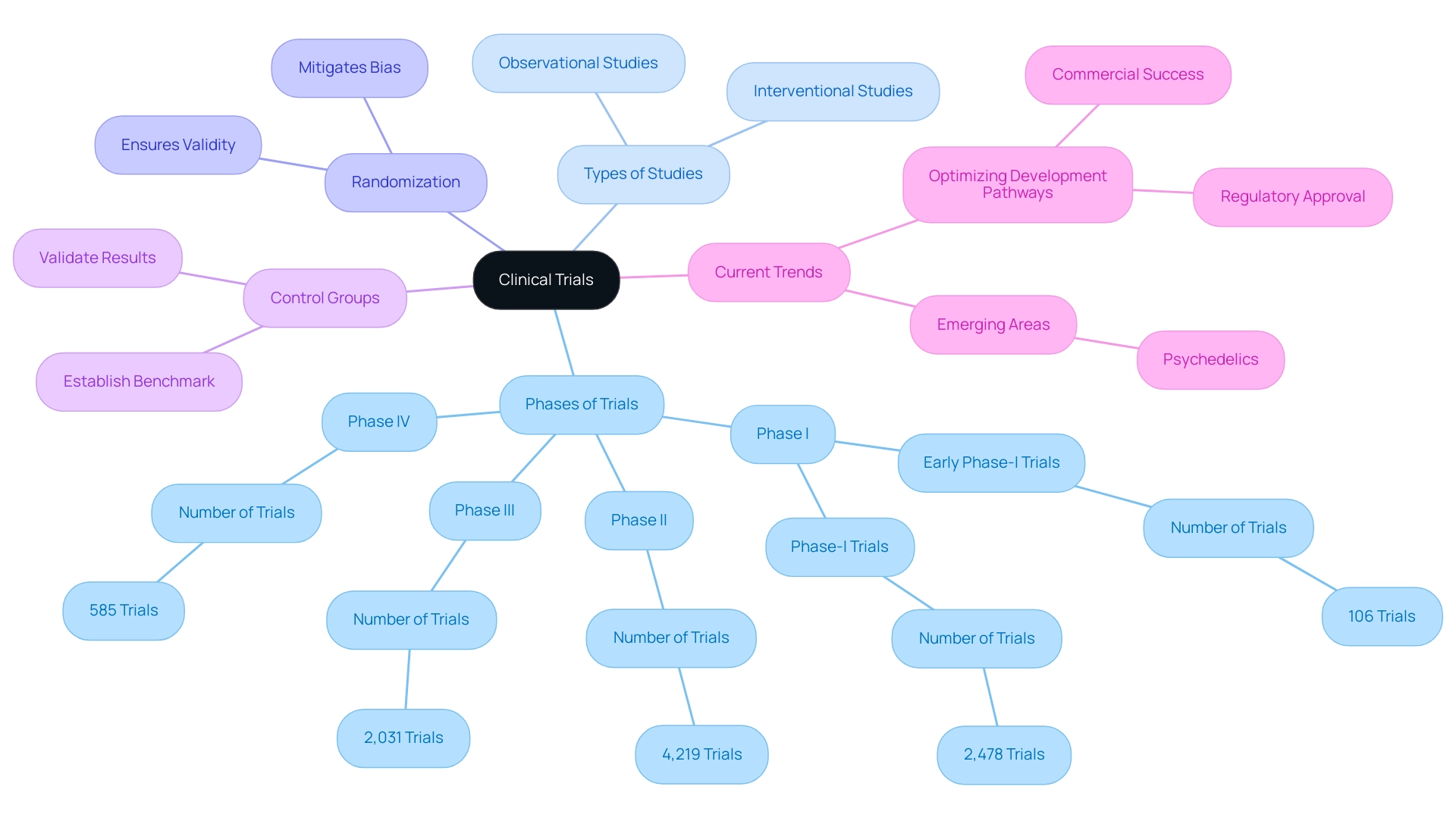
Phases of Clinical Trials: A Breakdown of Each Stage
Clinical studies are systematically categorized into four primary phases, each serving distinct purposes in the evaluation of medical devices.
Phase I: This initial phase primarily focuses on assessing safety and determining the appropriate dosage. Typically involving a small group of healthy volunteers, Phase I studies are crucial for identifying any adverse effects and establishing a safe dosage range. In 2022, there were approximately 2,478 active Phase I interventional studies, reflecting the ongoing commitment to safety in clinical research. Notably, bioaccess® excels in managing Early-Feasibility Studies (EFS) that align with this phase, leveraging over 20 years of expertise in Medtech to ensure thorough assessments.
Phase II: After successful Phase I assessments, Phase II studies examine the effectiveness of the apparatus while continuing to oversee safety. This phase often includes a larger cohort of participants, allowing researchers to gather more comprehensive data on the device's effectiveness. In 2022, there were approximately 4,219 active Phase II interventional studies, and real-world examples of Phase II efficacy evaluations have demonstrated promising outcomes, particularly in the context of innovative medical technologies. Bioaccess® supports these efforts with tailored methodologies that enhance study outcomes.
Phase III: This phase is pivotal as it compares the new intervention against standard treatments. Phase III studies typically involve a significantly larger participant pool, often in the thousands, to ensure robust data collection. The results of these tests are essential for regulatory approval and can affect medical guidelines. In 2022, there were 2,031 active Phase III interventional trials, highlighting the significance of this stage in the trial landscape. As highlighted by industry experts, homogenizing the use of specific outcomes across these phases can significantly enhance the development of clinical guidelines and facilitate future comparisons among interventions, as noted by Peng Lu, chief health officer of Dutch biotech Pharvaris. Bioaccess® is a vetted CRO and consulting partner for U.S. medical apparatus companies in Colombia, ensuring compliance and effective project management throughout this phase.
Phase IV: Once a device is approved and available for sale, Phase IV studies, or post-marketing surveillance, are carried out to monitor long-term effectiveness and safety in a broader population. This phase is essential for identifying any rare side effects that may not have been evident in earlier studies. In 2022, there were 585 active Phase IV interventional studies, emphasizing the ongoing need for monitoring after market entry. Bioaccess® also specializes in Post-Market Clinical Follow-Up Studies (PMCF), offering extensive assistance to guarantee ongoing safety and effectiveness.
Understanding the steps in designing device trials is crucial for creating effective evaluations that not only satisfy regulatory demands but also emphasize patient safety. In 2025, the average number of participants in research studies continues to evolve, with trends indicating a shift towards larger, more diverse groups to better reflect real-world scenarios. The environment of medical studies is dynamic, with ongoing updates and innovations influencing the methodologies used throughout all phases.
For instance, the Japanese market is becoming increasingly attractive to Western biopharmaceuticals due to regulatory changes aimed at expediting drug approvals, which is expected to enhance the availability of innovative therapies for patients. Moreover, progress in vascular medicine was emphasized by Dr. Jorge Hernando Ulloa, who presented one-year first-in-human data on the VenoValve® at the Charing Cross International Symposium, illustrating the significance of continuous research and development in this area.
In general, a thorough understanding of the stages of clinical studies is crucial for clinical researchers striving to create user-focused evaluations that effectively tackle both safety and efficacy.
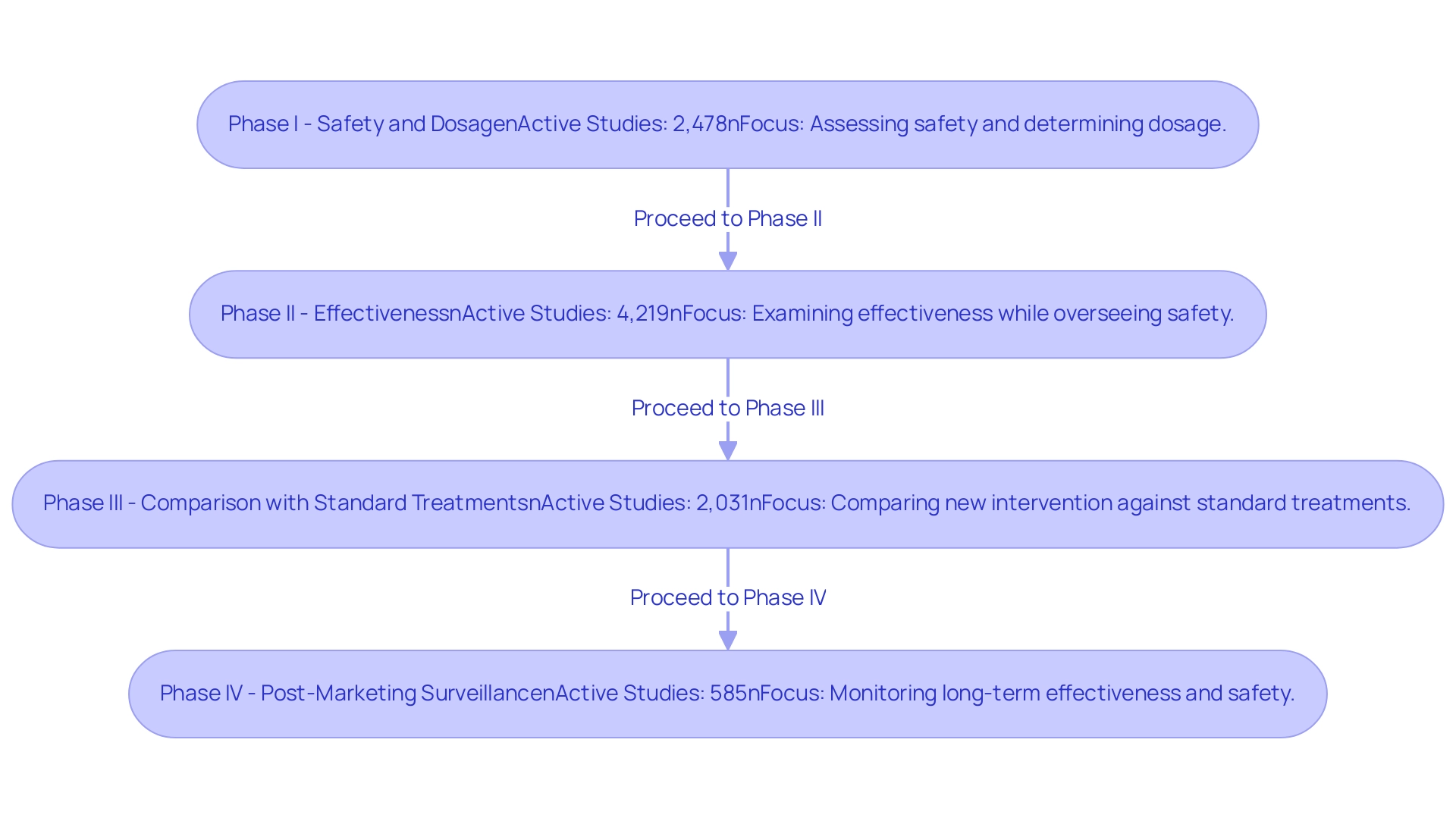
Special Considerations for Medical Device Trials
Medical equipment evaluations present unique challenges that necessitate comprehensive testing of safety and effectiveness in real-world conditions. Unlike drug studies, which primarily focus on pharmacokinetics, equipment evaluations must take into account various factors, including design, user interaction, and the specific characteristics of the patient population involved. In 2025, statistics indicate that nearly 70% of healthcare equipment assessments experience delays due to these complexities, underscoring the importance of meticulous planning and execution.
This is where extensive management services related to trials, such as those provided by bioaccess®, become crucial. They offer expertise in:
- Early-Feasibility Studies
- First-In-Human Studies
- Pilot Studies
- Pivotal Studies
- Post-Market Follow-Up Studies
Moreover, the regulatory environment for health products is exceedingly intricate, with pathways that can differ significantly from those for pharmaceuticals. Navigating the guidelines set forth by regulatory bodies like the FDA demands a thorough understanding of the nuances involved. Recent advancements, such as the introduction of single Institutional Review Board (sIRB) practices, aim to streamline the review process; however, they also require adjustments in standard operating procedures and resource allocation for many institutions.
A case study on regulatory innovation in clinical research highlights how the sIRB review seeks to reduce redundant evaluations in collaborative research, thereby accelerating study initiation while maintaining high standards.
Real-world testing is increasingly recognized as a vital component of healthcare product evaluations. The Galen Cloud, an innovative advancement in cloud-connected healthcare tools, exemplifies the shift towards integrating sophisticated technology to enhance testing efficiency and data management. This solution is designed to be quick, secure, robust, and user-friendly, making it an appealing option for researchers.
Expert opinions stress that the unique factors influencing health-related product evaluations extend beyond traditional testing criteria. For example, Danish Mairaj, principal engineer of healthcare equipment design at RESYCA, underscores the potential of artificial intelligence in analyzing vast datasets, provided relevant use cases are effectively implemented in suitable settings. This perspective aligns with the ongoing evolution of testing methodologies, which increasingly prioritize real-world relevance and participant safety.
In conclusion, the steps involved in designing device trials must address the inherent obstacles in clinical assessments by adopting a multifaceted strategy that encompasses thorough safety and efficacy testing, a solid grasp of regulatory frameworks, and innovative solutions to enhance assessment execution. With over 20 years of experience in Medtech, bioaccess® provides a tailored approach to navigate these complexities, particularly in the Latin American market. As the landscape continues to evolve, remaining informed about these dynamics will be essential for successful study design and implementation, especially with the support of experienced partners like bioaccess®.
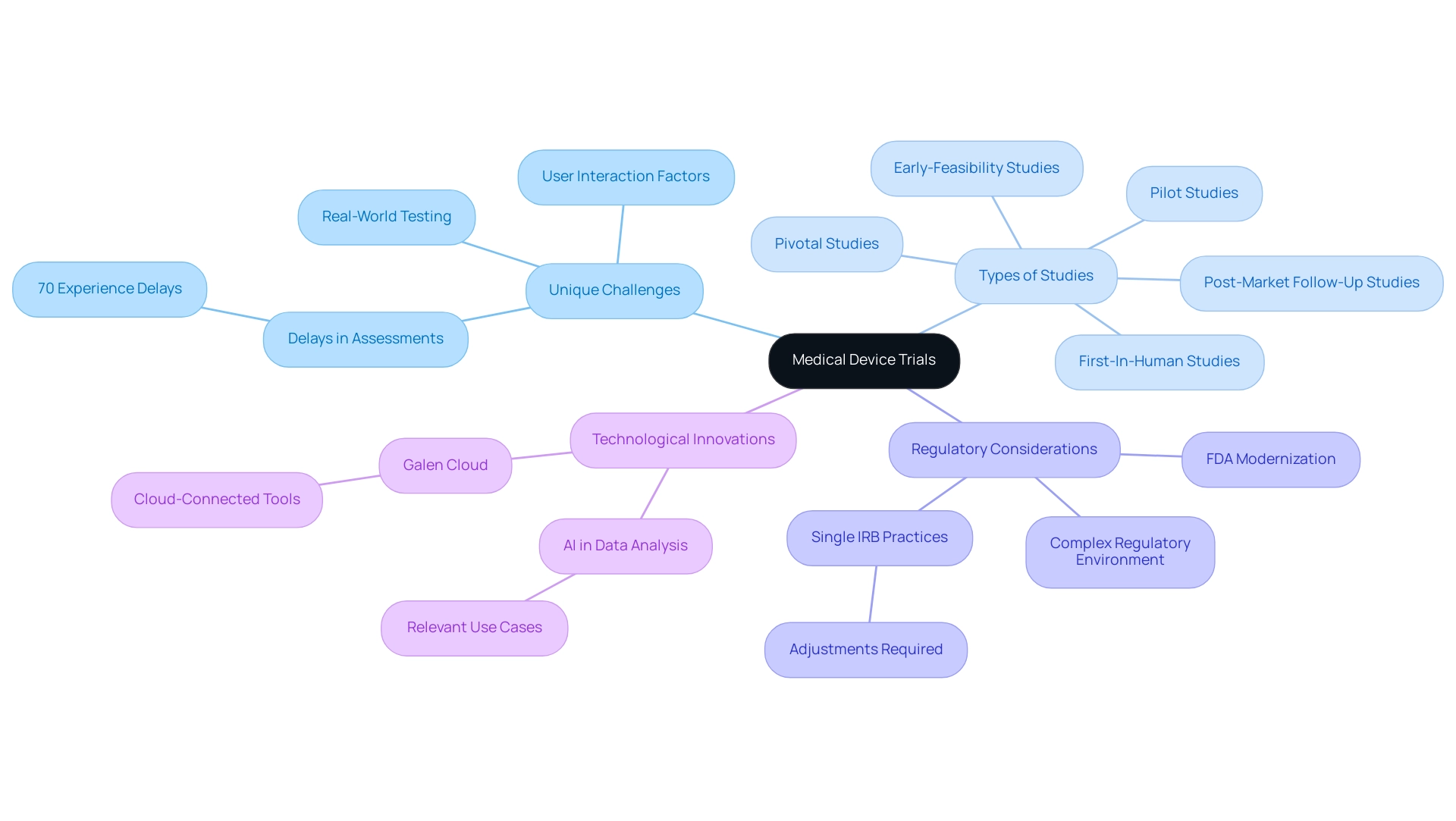
Navigating Regulatory Requirements in Device Trials
Navigating regulatory requirements is essential in the design of device trials for medical product studies. Regulatory bodies, particularly the FDA, have established comprehensive guidelines outlining the necessary steps for the approval of studies, including pre-market submissions and post-market surveillance. In 2025, the FDA continues to emphasize the importance of compliance, which not only safeguards participant safety but also accelerates the pathway for products to reach the market.
For instance, recent statistics indicate that successful pre-market submissions have increased by 15% compared to the previous year, reflecting a growing understanding of regulatory expectations among developers. To effectively manage device studies, clinical research professionals must understand the steps in designing device trials and be well-versed in the regulatory landscape. This encompasses grasping the nuances of FDA guidelines, which necessitate thorough documentation and adherence to protocols throughout the device trial design process. Bioaccess offers a comprehensive suite of clinical trial management services, including:
- Feasibility studies
- Site selection
- Compliance reviews
- Trial setup
- Import permits
- Project management
- Reporting
A notable case study highlights how a mid-sized medical technology company improved its regulatory compliance by implementing a compliance solution on the Atlassian Cloud, streamlining their processes and enhancing operational efficiency. Moreover, challenges in adverse event reporting—such as data quality, interoperability, and timeliness—are critical considerations in the design of device trials. Addressing these challenges is essential for ensuring that the design of device trials not only meets regulatory standards but also contributes valuable real-world evidence (RWE) derived from sources like electronic health records (EHRs) and patient registries. As Michelle E. Tarver, MD, PhD, stated, "Under our leadership, CDRH is enhancing efforts to include underserved and underrepresented populations in the assessment of health products," emphasizing the FDA's dedication to inclusivity in regulatory processes.
Furthermore, the recent FDA authorization of Guardant Health, Inc.'s Shield™ blood test for colorectal cancer screening serves as a timely example of successful navigation through regulatory requirements in the healthcare sector. As the terrain of healthcare equipment assessments evolves, remaining knowledgeable about FDA authorization procedures and recommendations will be vital for research professionals seeking to maneuver through these intricacies effectively. Reach out to discover how bioaccess can support your research requirements.
Effective Recruitment Strategies for Clinical Trials
Enrolling participants for research studies, particularly in the realm of medical device investigations, presents distinct challenges due to the necessity for specific patient groups. As we approach 2025, a significant shift is underway towards optimizing recruitment strategies that not only meet regulatory requirements but also ensure commercial viability. Effective recruitment strategies encompass a multifaceted approach, including the utilization of patient registries, collaboration with healthcare providers, and targeted advertising campaigns designed to reach potential participants.
Leveraging patient registries has emerged as a powerful tool in enhancing recruitment efforts. These registries grant access to a diverse pool of patients who may qualify for studies, thereby streamlining the onboarding process. Real-world examples illustrate that organizations employing patient registries have experienced marked improvements in participant engagement and retention rates.
For instance, bioaccess® has successfully utilized patient registries in its partnerships, notably with Caribbean Health Group, to enhance recruitment for clinical studies in Barranquilla, Colombia, establishing the city as a premier location for clinical research in Latin America. Remarkably, bioaccess® achieved over a 50% reduction in recruitment time while maintaining a 95% retention rate in its studies, underscoring the effectiveness of these strategies.
Furthermore, cultivating trust with potential participants is paramount. Providing clear, transparent information regarding the study's objectives, procedures, and potential benefits fosters a sense of security and encourages participation. In 2025, underrepresented study populations will have increased options for onboarding and visitations, emphasizing the significance of inclusive recruitment practices.
This inclusivity not only enhances participant diversity but also aligns with the growing emphasis on optimizing development journeys for both regulatory and commercial success.
Expert insights underscore the necessity of adapting recruitment approaches to the evolving landscape of research studies. As Max Baumann from Treehill Partners articulates, "We expect continued focus on optimizing the development journeys of assets to achieve not only an approval-enabling endpoint but to qualify for commercial success." This perspective highlights the imperative for early-stage developers to prioritize efficient recruitment as a core component of their research strategies.
Given the rising number of global research studies, addressing site capacity concerns is equally critical. Clinical research sites are grappling with challenges stemming from high staff turnover and complex protocols. A case study titled "Addressing Site Capacity Issues" illustrates that sponsors are anticipated to prioritize the standardization of site technology to enhance efficiency and rebuild site capacity for drug development.
By implementing these effective recruitment strategies, sponsors can bolster their chances of success in medical device studies—essential steps in designing device trials that ultimately lead to improved outcomes for patients and the healthcare system at large. Moreover, bioaccess® offers a comprehensive suite of study services, including Early-Feasibility Studies, First-In-Human Studies, Pilot Studies, Pivotal Studies, and Post-Market Follow-Up Studies, ensuring a tailored approach to meet the unique requirements of each investigation. The impact of these medical experiments extends beyond individual studies, contributing to local economies through job creation and healthcare enhancement.
Data Management and Analysis: Ensuring Trial Integrity
Data management and analysis are essential elements of clinical studies, serving to ensure that the data gathered is accurate, complete, and trustworthy. Establishing robust protocols for data collection, storage, and analysis is crucial, alongside implementing stringent quality control measures to mitigate errors. In 2025, the emphasis on data integrity has become increasingly pronounced, with statistics indicating that adherence to best practices can significantly enhance outcomes.
For instance, McKinsey estimates that the integration of predictive analytics could lower testing costs by 15-25%, making it an appealing strategy for sponsors and contract research organizations (CROs).
As emphasized by the ICH E6 R2 (and R3 revisions), sponsors are mandated to manage risk in a way that reduces the burden on sites, further underscoring the importance of effective data management practices. Comprehensive clinical study management services, such as those provided by bioaccess, encompass:
- Feasibility studies
- Site selection
- Compliance reviews
- Study setup
- Import permits
- Project management
- Reporting
These services are crucial in ensuring that studies are conducted efficiently and in compliance with regulatory standards, ultimately leading to improved outcomes and stakeholder satisfaction.
Statistical analysis plays a crucial role in interpreting the data, allowing researchers to draw valid conclusions regarding the safety and efficacy of medical devices. The significance of data integrity cannot be overstated; it is vital for maintaining the credibility of results and ensuring compliance with regulatory submissions. Recent trends indicate a shift towards consolidating data management processes in-house, as sponsors seek greater transparency and ownership of their data.
A significant case study showcasing effective data management practices is the application of blockchain technology in medical studies. This innovative approach facilitated secure and transparent data sharing among stakeholders, significantly enhancing data integrity and overall efficiency. The outcome demonstrated that blockchain not only improved trust among participants but also streamlined data management processes, showcasing its potential impact on the industry.
Furthermore, the influence of Medtech research studies extends beyond the experiments themselves, contributing to local economies through job creation, economic development, and healthcare enhancement. By fostering international collaboration, these studies drive global health improvement, ultimately benefiting communities worldwide.
Expert opinions emphasize the importance of stringent data management practices in medical studies. As Samruddhi Yardi rightly observed, "Through the dedication of volunteers and the commitment of researchers, research studies stand as a beacon of hope for a healthier, more resilient future, where healthcare knowledge continues to expand, and the quality of care consistently improves for individuals globally." By prioritizing data integrity and utilizing advanced methodologies, such as those offered by bioaccess, trials can attain more dependable results, ultimately benefiting the healthcare technology landscape.
Post-Market Clinical Follow-Up: Monitoring Device Performance
Post-market health follow-up is an essential component of the product lifecycle, focusing on the ongoing evaluation of a product's performance post-market approval. This process involves the systematic collection of data regarding adverse events, user experiences, and the overall effectiveness of the product in real-world applications. Regulatory agencies mandate post-market studies to ensure compliance and proactively address any emerging safety concerns.
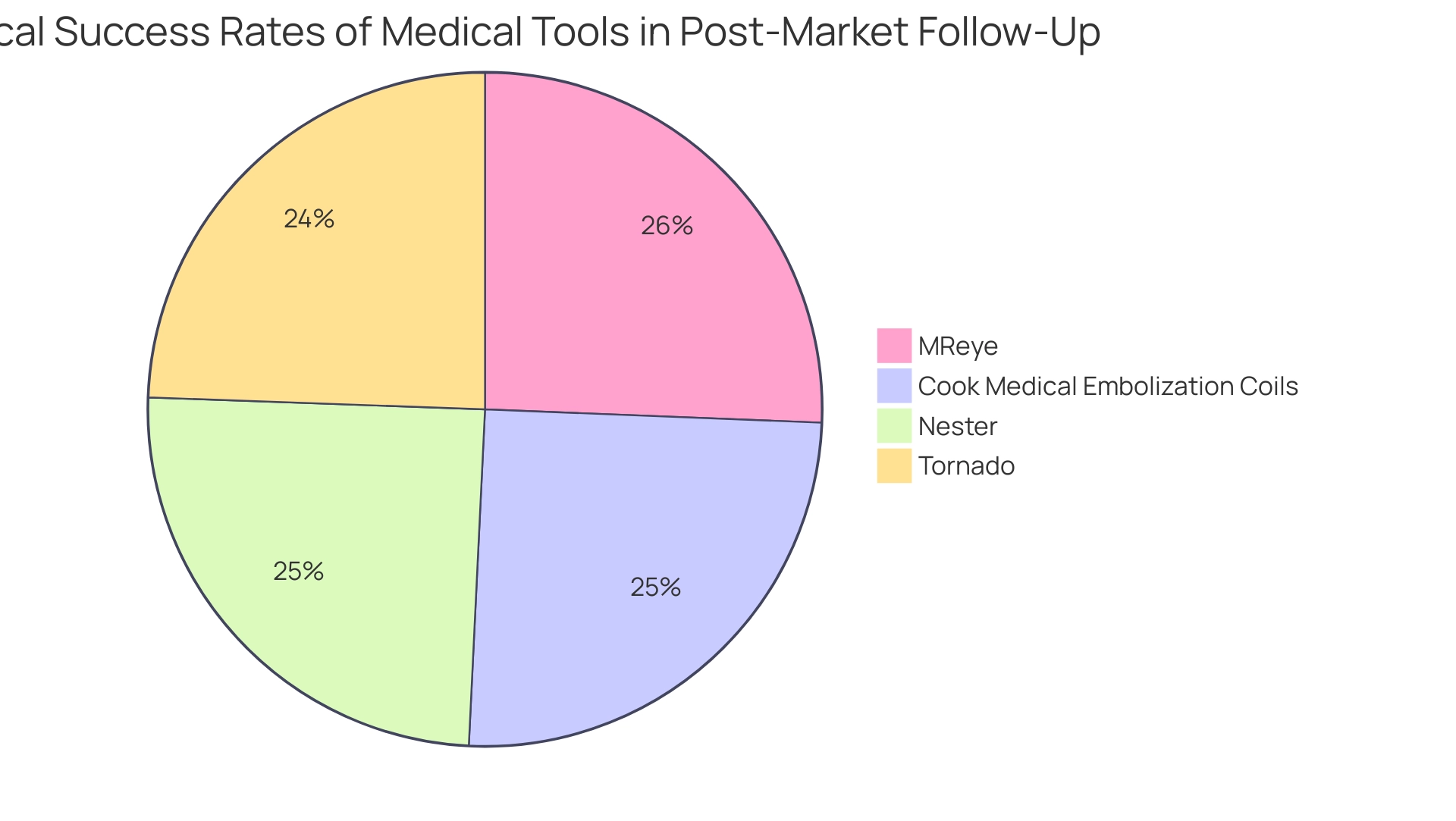
In 2025, the significance of post-market clinical follow-up is underscored by statistics indicating that technical success rates for various medical tools have reached remarkable levels, with organizations such as Nester achieving a rate of 95.4%, Tornado at 94.0%, and MReye at 98.6%. These figures highlight the effectiveness of rigorous post-market monitoring in upholding high performance standards and its vital role in user-centered trials.
Moreover, expert opinions assert that corrective and preventive actions (CAPAs) are crucial for addressing quality issues and preventing recurrence, as outlined in CFR 21 820.100. This regulatory framework ensures that manufacturers remain vigilant in monitoring performance, thereby enhancing patient safety and reliability. As Scott Snyder notes, "The ongoing assessment of device performance is not just a regulatory requirement; it is a commitment to the users who rely on these devices for their health and well-being."
Real-world examples further illustrate the importance of post-market medical follow-up. A notable case study involving embolization coils demonstrated the utility of real-world data (RWD) in supporting post-market safety and performance assessments. By examining medical data from Indiana's largest health system, researchers identified 323 patients treated with Cook Medical embolization coils, achieving a technical success rate of 96.7% and a safety events rate of 5.3%.
These outcomes not only met pre-established performance goals but also provided valuable longitudinal data for post-market surveillance (PMS) of healthcare instruments.
As the landscape of health instruments evolves, the necessity for thorough post-market research studies remains paramount. In 2025, ongoing performance monitoring after market approval is critical for ensuring that products continue to meet safety and efficacy standards, ultimately benefiting both manufacturers and patients alike. At bioaccess®, we leverage our 20+ years of expertise in overseeing post-market research follow-up studies, Early-Feasibility Studies, First-In-Human Studies, and Pilot Studies to ensure that your products are consistently evaluated for safety and effectiveness, aligning with regulatory requirements and enhancing user trust.
Our focus on Latin America enables us to provide tailored services that address the unique needs of this region. Engage with bioaccess® today to ensure your medical devices remain at the forefront of safety and innovation.
The Future of Clinical Trials: Trends and Innovations
The environment of medical studies is undergoing a significant transformation driven by technological advancements and innovative methodologies. By 2025, study sponsors are increasingly expected to implement a core outcome set across diverse study designs, highlighting a shift towards standardization that enhances the comparability and reliability of results. This expectation establishes a benchmark for quality and consistency in medical research.
Decentralized medical studies are gaining momentum, facilitating greater patient accessibility and involvement—an essential factor in today's fast-paced environment. Artificial intelligence (AI) is emerging as a crucial element in optimizing patient recruitment and data analysis. By harnessing AI, sponsors can more effectively identify and engage potential participants, ultimately improving enrollment rates and shortening timelines.
For example, recent case studies in the medical device sector illustrate that AI-driven recruitment strategies can significantly decrease the time needed to achieve enrollment targets, with some studies reporting reductions in enrollment timelines by as much as 30%.
Moreover, the integration of real-world evidence into research designs is becoming increasingly important. This approach enables researchers to gather insights from actual patient experiences, thereby informing trial methodologies and enhancing the relevance of findings. As Max Baumann from Treehill Partners asserts, "We expect continued focus on optimizing the development journeys of assets to achieve not only an approval-enabling endpoint but to qualify for commercial success."
This statement underscores the necessity for innovative strategies that not only fulfill regulatory requirements but also position products for commercial success in a competitive healthcare landscape.
Additionally, there is a growing trend among sponsors to insource data management processes and technologies, moving away from reliance on contract research organizations (CROs). This shift is driven by the need for enhanced control and transparency over medical data, ultimately improving data management quality and ensuring better patient outcomes.
As the medical research environment evolves, it is imperative for professionals to remain informed about these trends. The focus on refining development procedures, alongside regulatory approval, will be vital for achieving successful outcomes in medical studies. By embracing these innovations, clinical research organizations like bioaccess®, which specializes in comprehensive clinical trial management services—including Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Follow-Up Studies—can enhance operational efficiency and, in turn, improve patient care.
Conclusion
The exploration of clinical trials reveals their critical role in advancing medical interventions, ensuring both safety and efficacy through systematic methodologies. The distinct phases of trials, encompassing Phase I through Phase IV, alongside the unique challenges faced in medical device testing, contribute significantly to the overall success of clinical research. The importance of randomization, control groups, and adherence to regulatory requirements underscores the meticulous nature of these investigations, which are essential for achieving meaningful results.
As the landscape of clinical trials evolves, it becomes imperative to embrace emerging trends such as:
- Decentralized trials
- The integration of artificial intelligence
- The utilization of real-world evidence
These innovations not only enhance patient engagement and streamline recruitment but also ensure that clinical research remains relevant and aligned with contemporary healthcare needs. Furthermore, the focus on post-market clinical follow-up emphasizes the ongoing commitment to monitoring device performance and addressing safety concerns, reinforcing the principle that patient welfare is paramount.
In conclusion, understanding the intricacies of clinical trials is essential for researchers, developers, and stakeholders. By staying informed about the latest trends and innovations, and by collaborating with experienced partners, the clinical research community can continue to advance medical science, ensuring that new interventions are both safe and effective for patients worldwide. The journey of medical innovation is ongoing, and with each trial, the potential for improved health outcomes grows, ultimately benefiting society as a whole.
Frequently Asked Questions
What are clinical studies?
Clinical studies are systematic investigations designed to assess the safety and efficacy of medical interventions, including drugs and medical devices.
How are clinical studies organized?
Clinical studies are organized into distinct phases, each with specific objectives and methodologies.
What is the difference between interventional and observational studies?
Interventional studies actively assign participants to specific treatments, while observational studies monitor outcomes without direct intervention.
Why is randomization important in experimental studies?
Randomization is crucial because it mitigates bias, ensuring that observed treatment effects result from the intervention rather than confounding variables.
What were the statistics for active interventional studies in 2022?
In 2022, there were approximately: 1. 106 early phase-I interventional studies 2. 2,478 phase-I interventional studies 3. 4,219 phase-II interventional studies 4. 2,031 phase-III interventional studies 5. 585 phase-IV interventional studies.
What role do control groups play in clinical studies?
Control groups establish a benchmark against which the efficacy of interventions can be evaluated, which is essential for validating study results.
What services does bioaccess® provide for clinical studies?
Bioaccess® offers comprehensive management services, including feasibility assessments, site selection, compliance evaluations, setup, import permits, project oversight, and reporting.
What is the significance of the current trends in medical studies?
Current trends emphasize enhancing development pathways to secure regulatory approval and ensure commercial viability, particularly in emerging areas like psychedelics.
What are the phases of clinical studies?
Clinical studies are categorized into four primary phases: Phase I focuses on safety and dosage with a small group of healthy volunteers; Phase II examines effectiveness while continuing safety assessments with a larger cohort; Phase III compares the new intervention against standard treatments with a significantly larger participant pool; Phase IV conducts post-marketing surveillance to monitor long-term effectiveness and safety.
What are the benefits of understanding the stages of clinical studies?
Understanding the stages is crucial for creating effective evaluations that satisfy regulatory demands and emphasize patient safety.

