Introduction
The traditional institutional review board (IRB) review process plays a vital role in ensuring the ethical conduct of research. However, it is not without its challenges. Lengthy introductions and subjective evaluations can lead to delays, hindering research progress.
In response, expedited IRB reviews offer a streamlined approach for low-risk studies, reducing approval time and facilitating efficient research. This article explores the benefits of expedited reviews, key strategies for streamlining the IRB process, and the importance of addressing ancillary reviews and compliance issues. Additionally, it highlights best practices for minimizing delays and presents case studies that illustrate successful implementation of expedited IRB review.
By embracing these approaches, researchers can navigate the complexities of the IRB process and contribute to the advancement of medical knowledge while upholding ethical and regulatory standards.
Challenges in Traditional IRB Review Processes
The institutional board process is a cornerstone of ethical investigation, safeguarding the well-being of participants and ensuring adherence to established standards. However, the conventional IRB assessment can be burdened with inefficiencies, such as lengthy introductions, now nearly fivefold longer than in the 1970s, that slow down the evaluation process. These cumbersome procedures often lead to delays, stalling pivotal research advancements.
In the context of IRBs, the intricate web of reviews and the subjective nature of evaluations can cause substantial lags. For instance, proposals submitted for IRB consideration provide information about the research's objectives, methods, potential risks and benefits, and consent forms. Yet, the assessment of these elements is not always straightforward, as it involves judgment calls on ethical matters and participant consent, reflecting a process that can be as nuanced as it is critical.
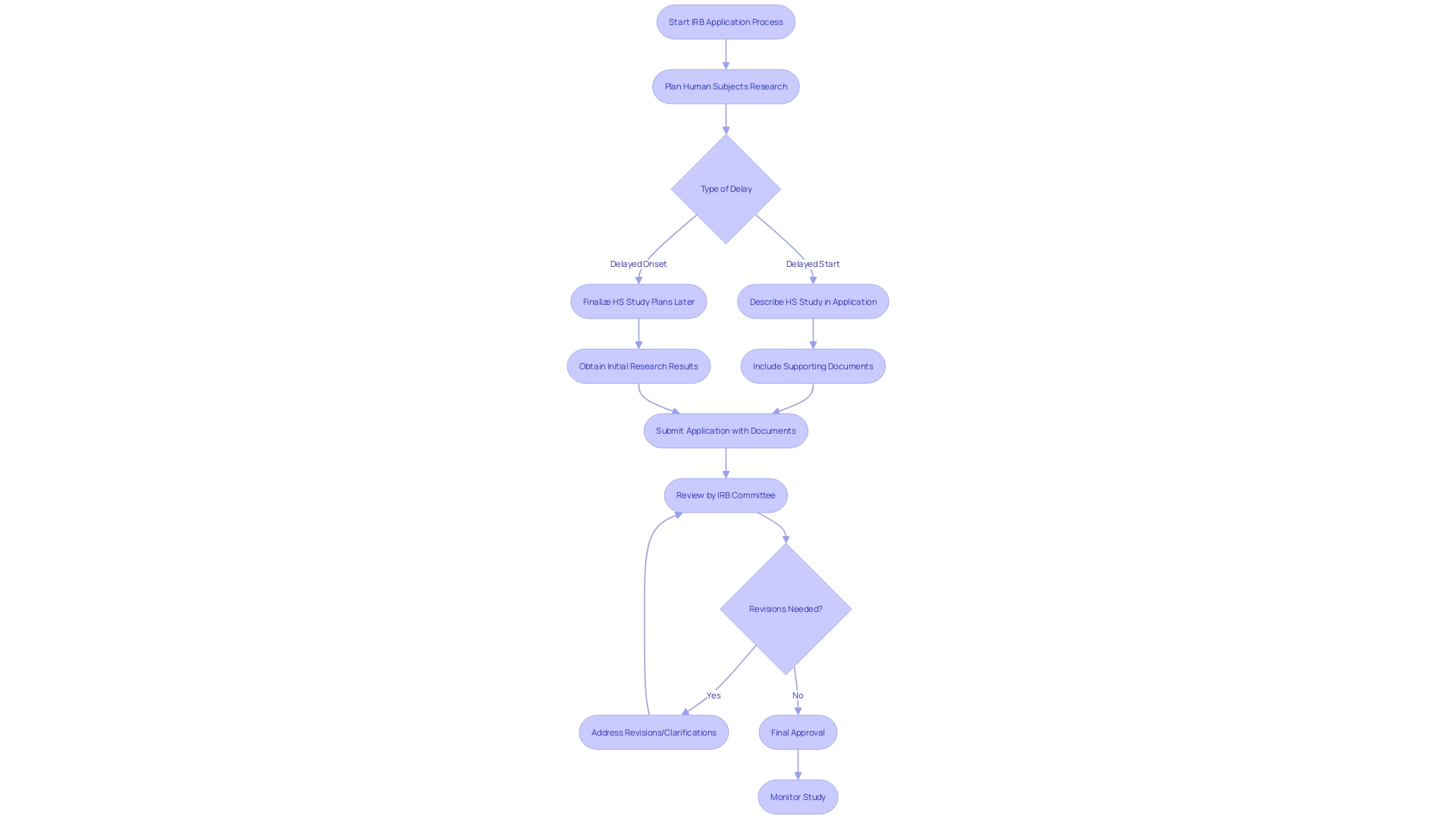
Statistics highlight the consequences of these delays. A clear differentiation is made between 'delayed start' and 'delayed onset' in human subjects studies. The first option permits a comprehensive plan for investigation within the application, whereas the second option, frequently misinterpreted, necessitates initial outcomes to delineate the research, which could result in unfavorable evaluation or funding obstacles.
Moreover, the real-life application of ethics requirements, such as personal data protection, can be narrower than anticipated, complicating the researchers' task to meet these guidelines. These challenges highlight the significance of modifying IRB procedures to streamline the assessment process without jeopardizing the moral integrity of investigation.
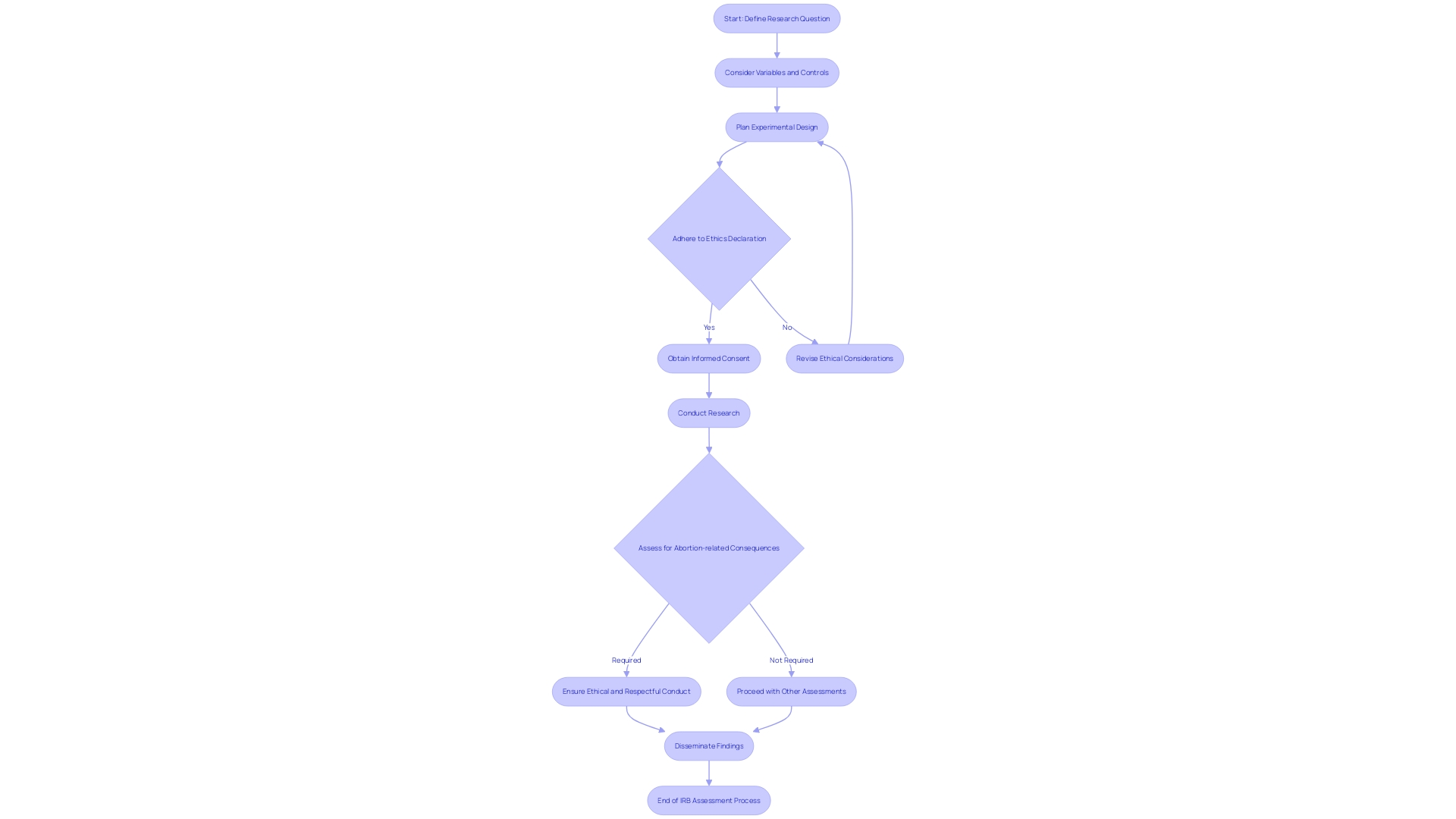
What is Expedited IRB Review?
The procedure of accelerated Institutional Review Board (IRB) evaluations provides a simplified method for specific study suggestions, especially when the investigation poses minimal danger to participants. This method involves a select group of IRB members or a single designated reviewer assessing the proposal, rather than convening the full board. The objective is to protect human participants while also removing needless delays in the timeline of investigation.
Expedited evaluations are suitable for studies involving procedures that are both well-established and have a proven track record of safety. This is in contrast to more intricate investigations that necessitate the examination of the entire board to guarantee that every aspect of the exploration adheres to rigorous ethical standards. These less risky studies, therefore, benefit from a faster approval process, allowing investigation to commence sooner and facilitating a more efficient path to valuable findings.
To illustrate the significance of this process, consider the analogy of safety standards for utilities in California, where stringent evaluations are conducted to mitigate the risk of catastrophic wildfires. In the same way, accelerated IRB evaluations serve a vital role in guaranteeing responsible conduct of studies, without creating unnecessary obstacles to advancement.
Moreover, the expedited review can be particularly beneficial when time is of the essence, such as during pandemics where vaccine development is accelerated through phases to establish safety and efficacy. The goal is to harmonize the rigorous standards of research with the urgency of delivering timely healthcare interventions.
For researchers navigating the intricacies of IRB applications, it's vital to grasp the distinction between delayed start and delayed onset investigations. A delayed start study will commence later within the funding period and requires a full description in the application, whereas a delayed onset study cannot be fully defined at the time of application and depends on preliminary results to finalize the human subjects study plans.
Ultimately, expedited IRB evaluations represent a critical balance between the imperative to protect study participants and the need to advance medical knowledge swiftly and efficiently.
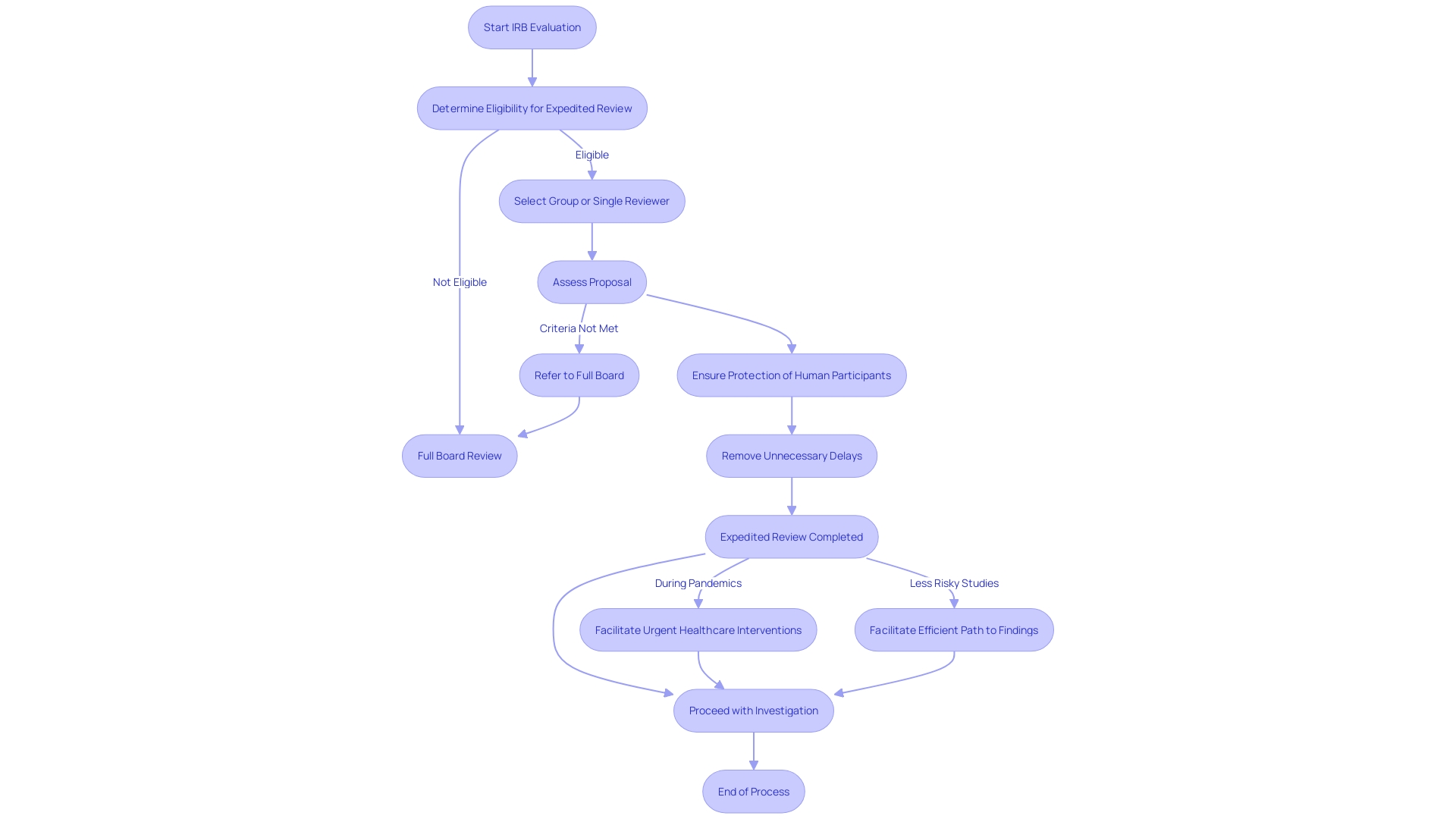
Benefits of Expedited IRB Review
The expedited Institutional Review Board (IRB) process presents numerous advantages, particularly in streamlining the approval of study proposals. One of the primary benefits is the significant reduction in time to receive IRB approval. By circumventing the comprehensive board assessment, which frequently requires organizing multiple gatherings, researchers can obtain endorsement for their investigations more quickly. This expedited process is particularly advantageous when research needs to be initiated quickly to take advantage of time-sensitive opportunities.
Moreover, the accelerated evaluation procedure is enhanced to concentrate on research that presents minimal risk and employs well-established methods. This strategic allocation of IRB resources ensures that research projects with lower risk profiles do not overly burden the evaluation process. Additionally, it facilitates a more efficient use of time and resources, allowing IRBs to concentrate on more complex protocols that require detailed scrutiny.
Finally, an accelerated evaluation procedure promotes a synergistic connection between researchers and the IRB. This partnership leads to a more streamlined and impactful supervision system, ultimately benefiting the wider domain of investigation by allowing inquiries to begin promptly while upholding ethical and regulatory requirements.
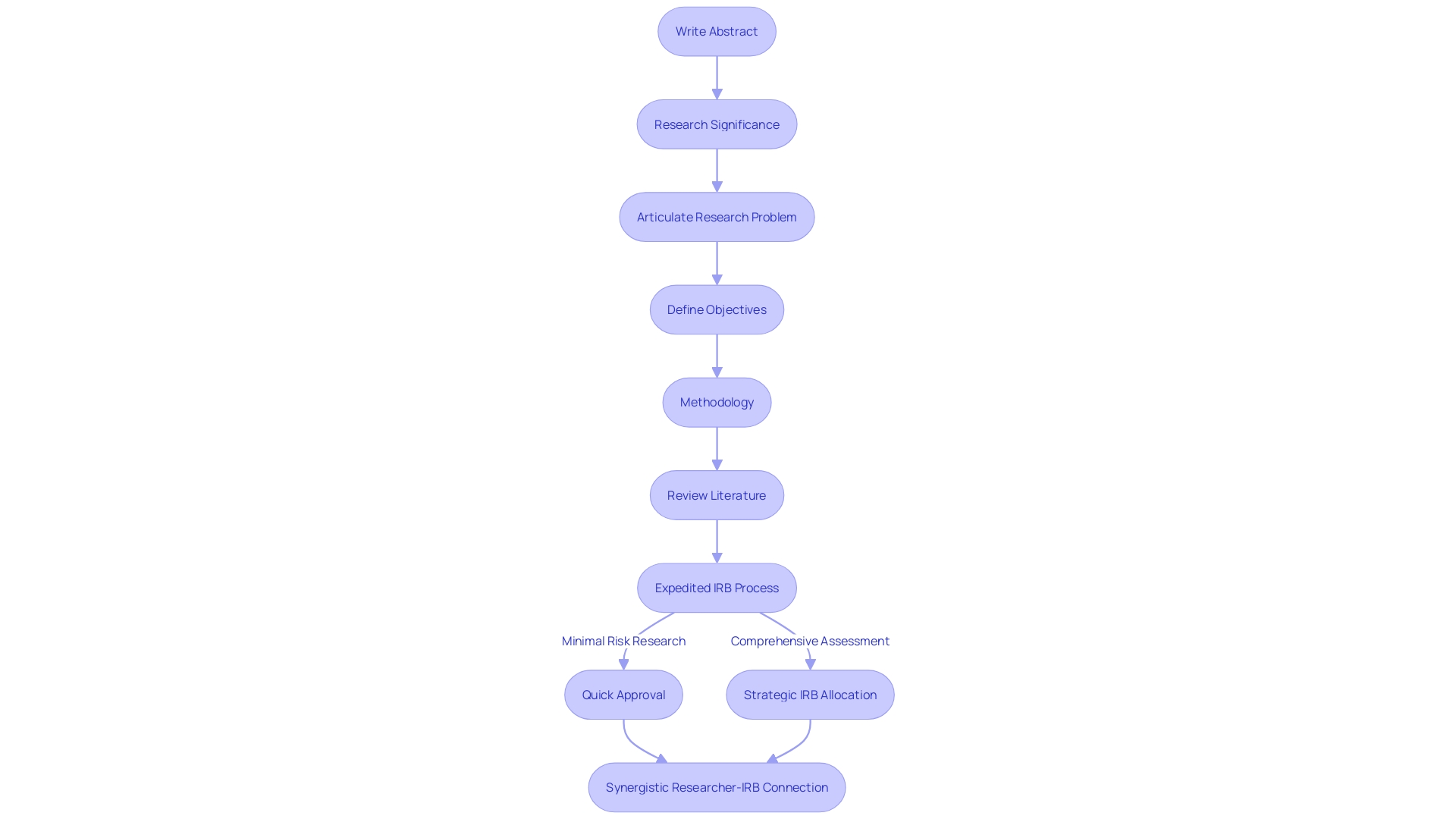
Key Strategies for Streamlining IRB Review
Maximizing the IRB assessment procedure is essential for the prompt initiation and conclusion of vital research investigations. A cooperative method involving early involvement of investigators and IRB members promotes a mutual comprehension of the research's goals and proactive identification of potential problems. Implementing a pre-review checklist ensures all documentation meets IRB requirements, thus reducing potential delays. Centralized, real-time IRB platforms streamline the submission, review, and communication process, enhancing efficiency and reducing document handling times. Such strategies are aligned with the ethical oversight provided by IRBs, informed by historical precedents and the diverse composition of committee members from various backgrounds, as mandated by federal regulations and ethical guidelines, such as the Belmont Report. This is imperative, as the stakes are high; thousands of volunteers participate in clinical trials annually, often without direct benefit, justified by the broader scientific knowledge gained. Technological advancements, such as the PressureSafe device by IR-MED, demonstrate the continuous requirement for IRB attentiveness as products undergo clinical usability assessments and await FDA clearance. The distinction between postponed initiation and postponed commencement in proposals is also crucial, guaranteeing precise depiction of the timeline and avoiding adverse effects on the evaluation of the application. In general, these strategies and considerations constitute a thorough approach to accelerate IRB processes, in line with the ethical and regulatory frameworks that protect participant rights and the integrity of investigations.
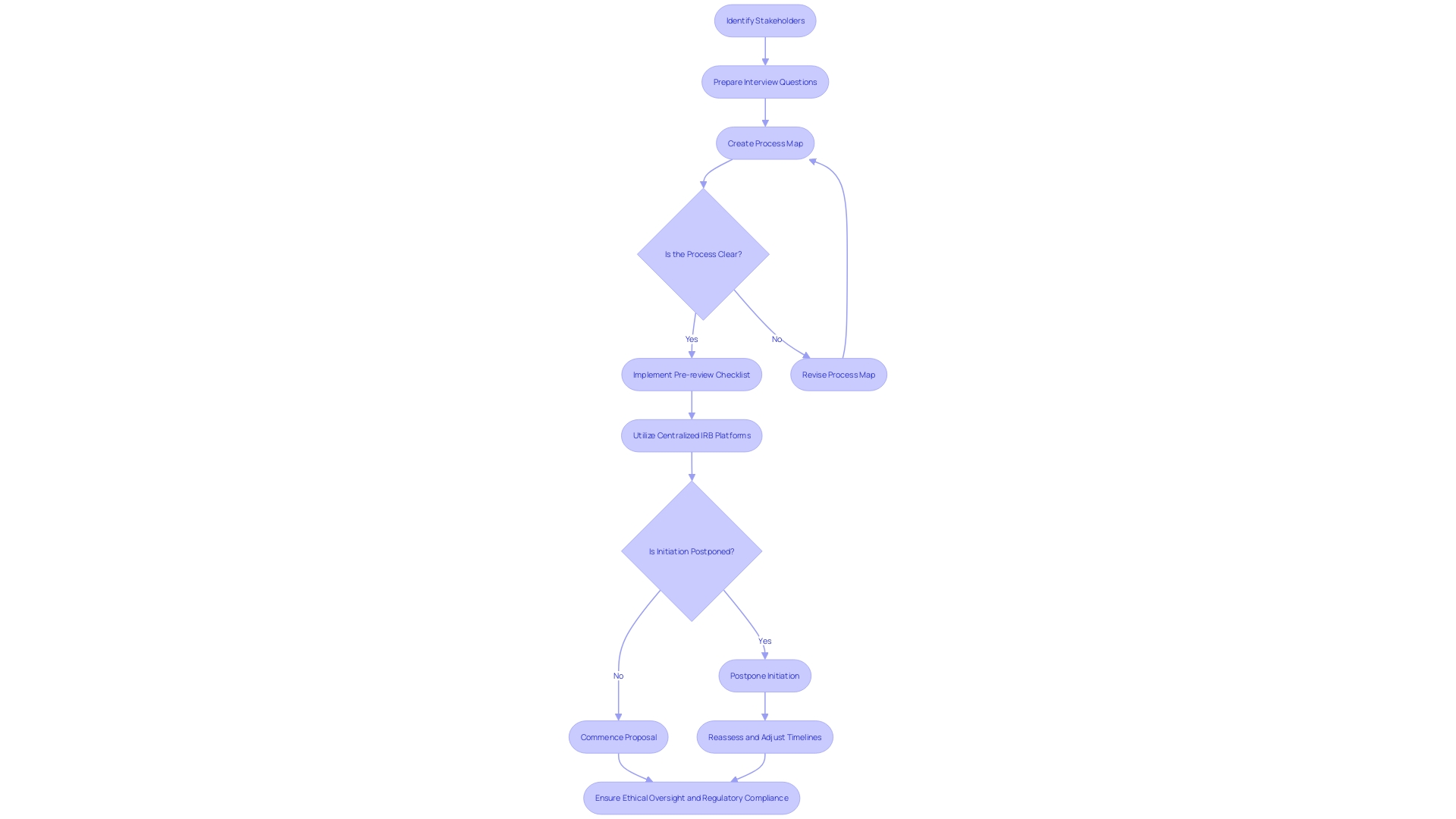
Addressing Ancillary Reviews and Compliance Issues
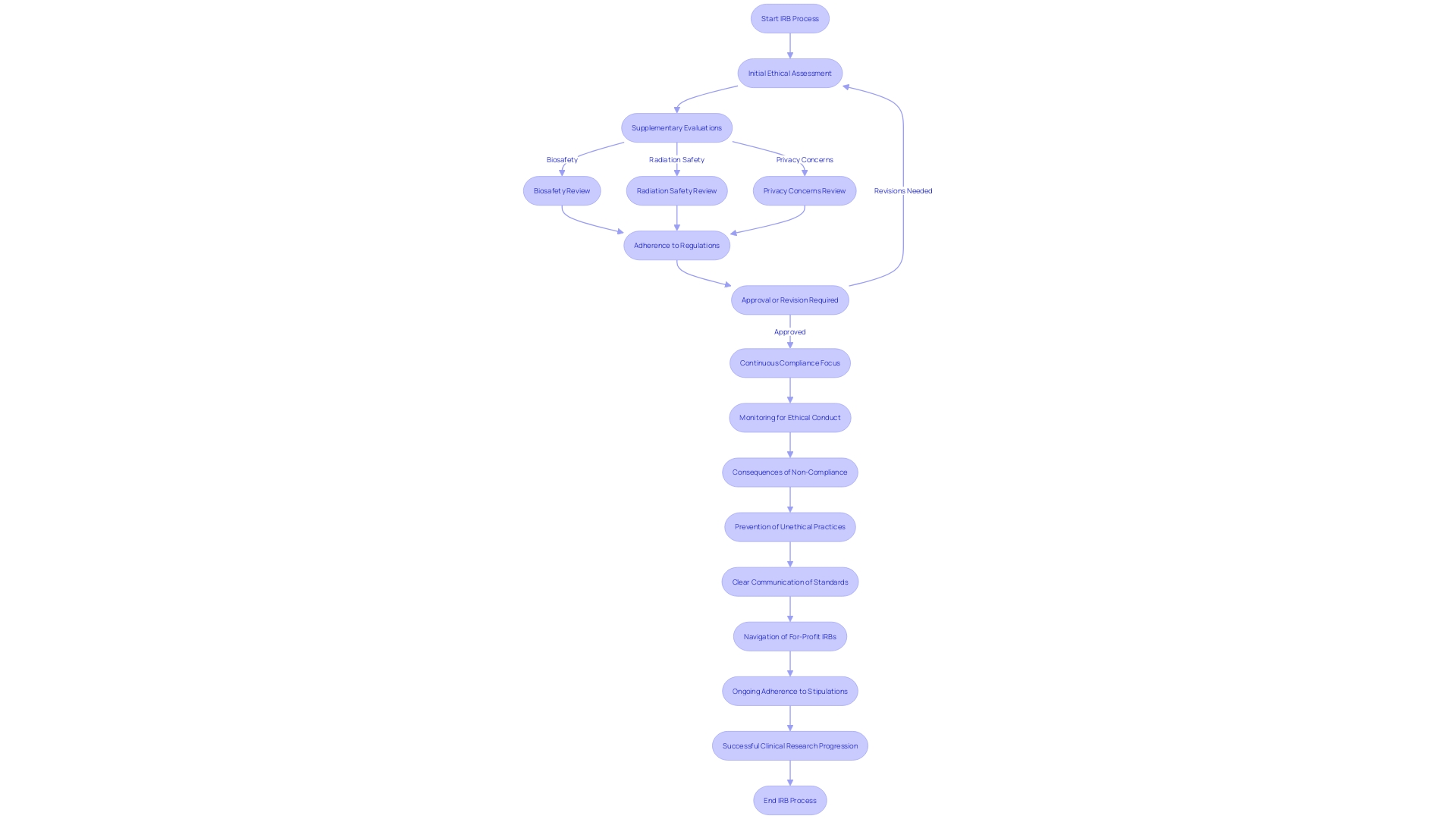
When submitting an Investigational New Drug (IND) application, a thorough and punctilious approach to Institutional Review Board (IRB) processes is paramount. This involves not just the main ethical assessment but also dealing with additional evaluations and adherence concerns that are crucial for the seamless advancement of research projects. These supplementary evaluations may relate to biosafety, radiation safety, or privacy concerns and are dependent on the nature of the study. A proactive approach to recognize and address these evaluations is crucial in avoiding delays and maintaining compliance with all relevant regulations.
Moreover, compliance is not a mere checkpoint but a continuous focus throughout the review process. Scientists must meticulously follow ethical guidelines, data protection protocols, and regulatory requirements to maintain the integrity of their studies and uphold the trust of stakeholders. As demonstrated by historical precedents like the Tuskegee Syphilis Study, the outcomes of non-compliance or ethical lapses in investigation can be severe, prompting the creation of IRBs to prevent such unethical investigation practices.
The FDA's recent publication of standards for direct-to-consumer prescription drug advertisements underscores the importance of conveying information in a manner that is clear, conspicuous, and neutral. This guidance parallels the necessity for researchers to communicate their adherence to ethical and compliance standards in their protocols and submissions to IRBs, ensuring that the overarching goal of safeguarding public health is upheld.
Additionally, scholars should be aware of the differences between postponed initiation and postponed beginning terminologies when strategizing their proposals. A misstep in this area could result in missing crucial details in the application, potentially negatively impacting the application's evaluation or resulting in a Bar to Award.
The current landscape, with nearly half of IRBs being for-profit and serving private companies, introduces an additional layer of complexity. Researchers must navigate this landscape with acuity, ensuring their proposals meet not only federal regulations but also additional criteria that may be imposed by these IRBs.
In the context of IND applications, it is essential to recognize that the IRB's role extends beyond the initial approval. Continued adherence to IRB stipulations and maintaining a proactive stance on supplementary assessments are essential for the successful progression of clinical research. With this comprehensive approach, researchers can bypass potential barriers, such as prior authorization problems that have been reported by a significant percentage of insured adults, and avoid delays similar to those observed in the examination of sensitive or classified information in government reports.
Best Practices for Minimizing Delays
To improve the effectiveness of the accelerated IRB evaluation process, it is crucial to implement a sequence of strategic measures. Clarity and precision in the documentation of the research proposal are foundational. Offering a thorough and well-organized proposal expedites the evaluation process by minimizing inquiries or the requirement for supplementary information. Moreover, active and timely communication with the IRB is paramount. Responding swiftly to IRB inquiries and making necessary revisions promptly can avert undue holdups. Additionally, engaging regulatory experts or consultants can offer substantial benefits. Their specialized knowledge and advice can help navigate the complexities of the IRB review, ensuring adherence to regulations and reducing the likelihood of delays.
Grasping the intricacies of 'delayed start' versus 'delayed onset' is also crucial for researchers planning investigations involving human subjects that won't commence immediately. An 'extended beginning' permits the complete depiction of the investigation on human subjects in the application with all relevant paperwork, while 'delayed initiation' implies that specifics of the inquiry on human subjects cannot be entirely outlined during the application stage and will be established according to preliminary findings. Mislabeling these terms can have serious repercussions on the application's evaluation.
Furthermore, it's vital to recognize the gravity of thorough IRB scrutiny. The IRB's role in upholding ethical standards in research is underscored by historical precedents, such as the Tuskegee Syphilis Study, which highlight the catastrophic outcomes of unethical research practices. The IRB process, established to prevent such incidents, requires detailed proposals that outline the research's purpose, methodology, risks, benefits, and consent forms. The review process may necessitate revisions, which emphasizes the importance of researchers being prepared for potential back-and-forth discussions.
Current developments in medical technology, such as the development of PressureSafe by IR-MED Inc., illustrate the importance of innovation in healthcare and the role of IRB in ensuring the ethical deployment of such technologies. PressureSafe, a handheld optical monitoring device for early detection of pressure injuries, is undergoing usability studies and showcases the ongoing efforts to advance medical devices while adhering to rigorous ethical standards.
In light of these considerations, researchers must be meticulous in planning and preparing their IRB submissions to minimize delays and contribute to the advancement of medical knowledge and patient care.
Case Studies: Successful Implementation of Expedited IRB Review
Real-world applications of expedited IRB processes are essential to comprehend for those involved in clinical studies. An important instance of such application is the differentiation between 'delayed start' and 'delayed onset' within human subjects investigation. The previous relates to investigation that won't start right away but is scheduled for later in the funding duration with all human subjects details provided upfront. The latter, however, suggests that the details of the human subjects investigation cannot be completely determined at the time of application and requires preliminary findings for subsequent planning. Furthermore, navigating the complexities of IRB approval is underscored by the case of IR-MED Inc., which is developing the PressureSafe device to non-invasively monitor pressure injuries. Their journey highlights the clinical research stages and the regulatory landscape they must traverse, including securing patents, conducting usability investigations, and awaiting FDA approval.
Another compelling case study involves the sensitive nature of investigation in national security, where investigators must secure government security clearances to work on classified programs. This underscores the additional layers of consideration required for IRB processes involving sensitive information. Furthermore, the attentiveness to plagiarism in grant applications, as demonstrated by the way NIH deals with such cases, is a vital element in preserving the integrity of proposals and the assessment process by peers.
These examples not only offer insights into the practicalities of accelerated IRB assessments but also showcase the broader implications and challenges that may arise, such as the necessity for confidentiality and adherence to ethical standards. As the research landscape evolves, so too must the strategies and approaches employed to streamline the IRB review process, as illustrated by these case studies.
Conclusion
In conclusion, expedited IRB reviews offer a streamlined approach to the traditional review process, reducing delays and facilitating efficient research. Researchers can navigate the complexities of the IRB process while upholding ethical and regulatory standards.
The benefits of expedited reviews include a significant reduction in approval time, allowing researchers to seize time-sensitive opportunities. This process focuses on low-risk studies, optimizing resource allocation and enabling a more efficient path to valuable findings. It also fosters a collaborative relationship between researchers and the IRB, enhancing oversight effectiveness.
To streamline the review process, key strategies can be implemented. Early engagement of investigators and IRB members promotes understanding and proactive issue identification. A pre-review checklist ensures compliance, minimizing potential delays.
Centralized, real-time IRB platforms improve efficiency and communication, aligning with ethical oversight and regulatory frameworks.
Addressing ancillary reviews and compliance issues is crucial for research progress. Researchers must proactively tackle biosafety, radiation safety, and privacy concerns. Ongoing compliance with ethical guidelines and regulatory requirements preserves research integrity.
Understanding the distinctions between delayed start and delayed onset studies is essential.
Best practices for minimizing delays include clear and comprehensive research proposals, timely communication with the IRB, and engaging regulatory experts. Thorough IRB scrutiny upholds ethical standards and prevents unethical practices. Real-world case studies highlight successful implementation, emphasizing confidentiality, adherence to ethical standards, and evolving strategies.
In conclusion, expedited IRB reviews streamline the review process while maintaining ethical and regulatory standards. By implementing key strategies and learning from real-world examples, researchers can navigate the complexities of the IRB process and contribute to the advancement of medical knowledge.




