Introduction
In the world of clinical research involving human subjects, Institutional Review Boards (IRBs) play a crucial role in ensuring the protection of participants and upholding ethical standards. These boards review and approve research protocols, carefully considering the risks and benefits faced by volunteers. However, the review process can be complex, with different levels of scrutiny depending on the nature of the study.
In this article, we will explore the purpose and function of IRBs, the different types of IRB reviews, what qualifies for expedited review, the benefits of expedited review, strategies to streamline the IRB process, the role of collaboration in reducing turnaround time, the concept of real-time IRB review, addressing common challenges in expedited reviews, and best practices for optimizing expedited review. By understanding these aspects, researchers can navigate the IRB process more efficiently, ensuring the integrity and quality of their research while safeguarding the well-being of participants.
The Purpose and Function of IRBs
Comprehending the accelerated review procedure for Institutional Review Boards (IRBs) is vital, especially in the context of clinical studies involving human subjects. IRBs play a pivotal role in ensuring the protection of human subjects by reviewing and approving study protocols. Their diligence is vital, as thousands of individuals volunteer for clinical trials every year, often without the prospect of direct benefits. IRBs are tasked with justifying the risks and burdens faced by volunteers, not through potential direct benefits, but through the scientific knowledge that may benefit future patients.
The necessity for an expedited IRB evaluation becomes evident when considering the rigorous assessment required to evaluate the scientific merit of investigation protocols. This includes scrutinizing the rationale of the study, the scientific background, and the feasibility of the approach. Significantly, advancements in the investigation may impact the significance of the suggested studies. However, even without novel concepts, a project can be critical for the field, underscoring the need for a nuanced review process.
In the wider perspective, the intricacy of risk assessment in swiftly developing areas, like the possible incorporation of AI in clinical studies, mirrors the difficulties experienced by California's utilities in reducing wildfire risks. These challenges include uncertain risk levels, unclear cost-effectiveness of mitigation strategies, and reputational risks amplified by media and political discourse. Likewise, the ethics of transplanting human brain organoids into animals emphasizes the developing nature of investigation and the significance of proactive ethical management.
Moreover, researchers must navigate terms like 'delayed start' and 'delayed onset' when planning studies involving human subjects, with each term carrying specific implications for IRB submissions. Not understanding these differences could result in incomplete applications and possible obstacles in the approval phase.
Considering the enhanced focus on ethics in NeurIPS 2023 Ethical Review, the prominence of IRBs in upholding ethical standards becomes more evident. Their oversight is vital in upholding the integrity of clinical trials and the welfare of human subjects. Therefore, a fast-tracked IRB evaluation procedure must strike a balance between effectiveness and the thorough assessment required to uphold ethical practices in the field of study.
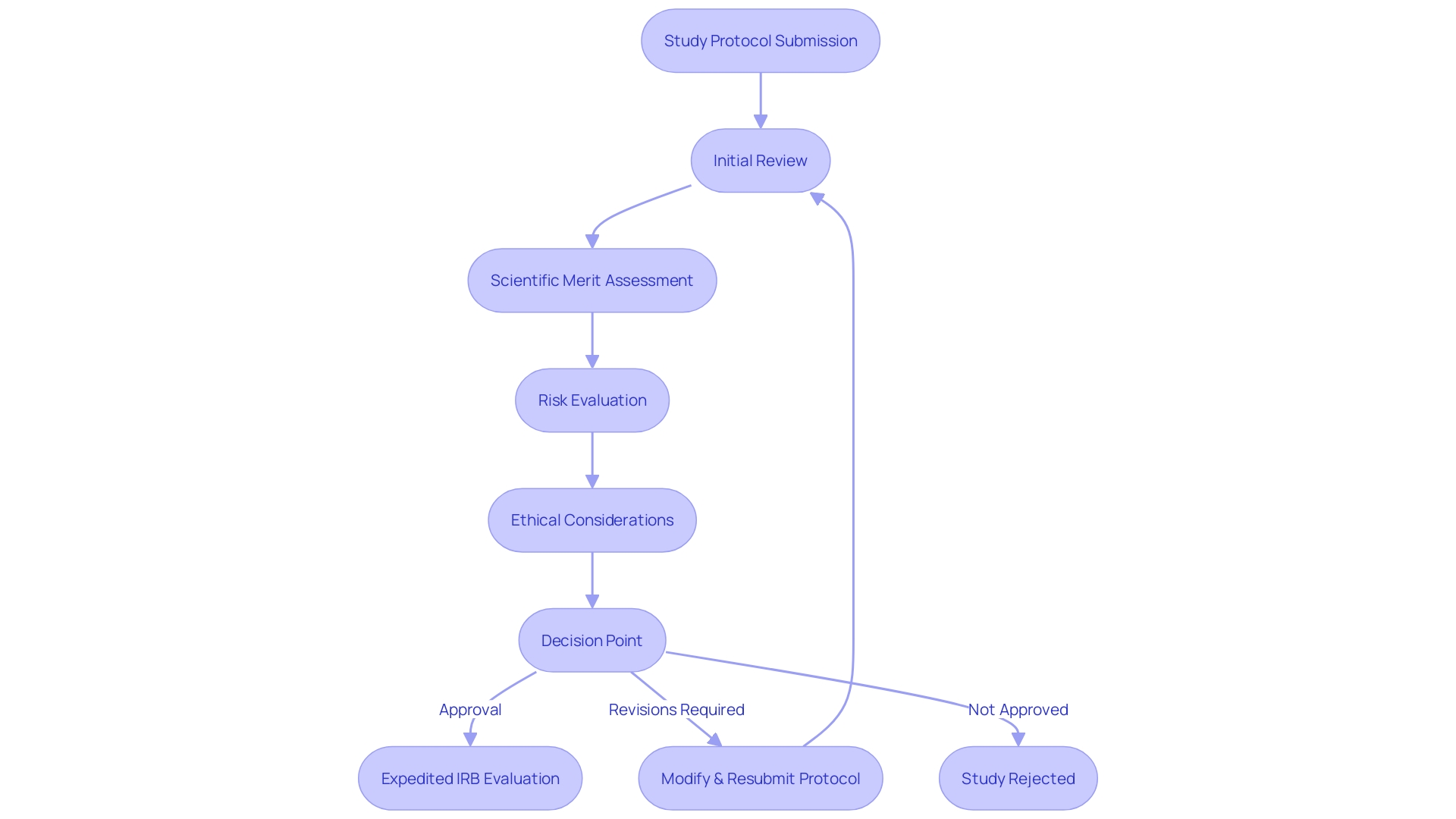
Types of IRB Review: Full Board, Expedited, and Exempt
Comprehending the levels of Institutional Review Board (IRB) scrutiny is crucial for efficiently navigating proposals, especially those linked to Investigational New Drug (IND) applications. The IRB's role is to safeguard participants, ensuring that the benefits of research outweigh the risks. There are three primary levels of IRB assessment: full board, expedited, and exempt.
A complete board assessment is compulsory for studies presenting greater than minimal risk to subjects or when delicate subject matter is involved. In this case, each member of the IRB is required to engage in the assessment procedure, contemplating the ethical and regulatory compliance of the suggested investigation.
Expedited evaluation is an IRB procedure for certain kinds of research involving no more than minimal risk, and for minor changes in approved research. It is not a faster iteration of a full board assessment but an evaluation by one or more experienced IRB members. This procedure is crucial for investigations that exhibit minimal danger and satisfy specific requirements, enabling researchers to progress without the necessity of complete board evaluation.
Lastly, exempt assessment pertains to investigation undertakings that involve negligible risk and fit into specific categories defined by federal regulations. Although exempt, such projects must still be submitted for IRB verification of the exemption status to ensure all ethical standards are maintained.
These distinctions are crucial for researchers to understand as they align their study designs with the appropriate level of IRB assessment, streamlining the procedure and facilitating a smoother path to commence vital investigation activities.
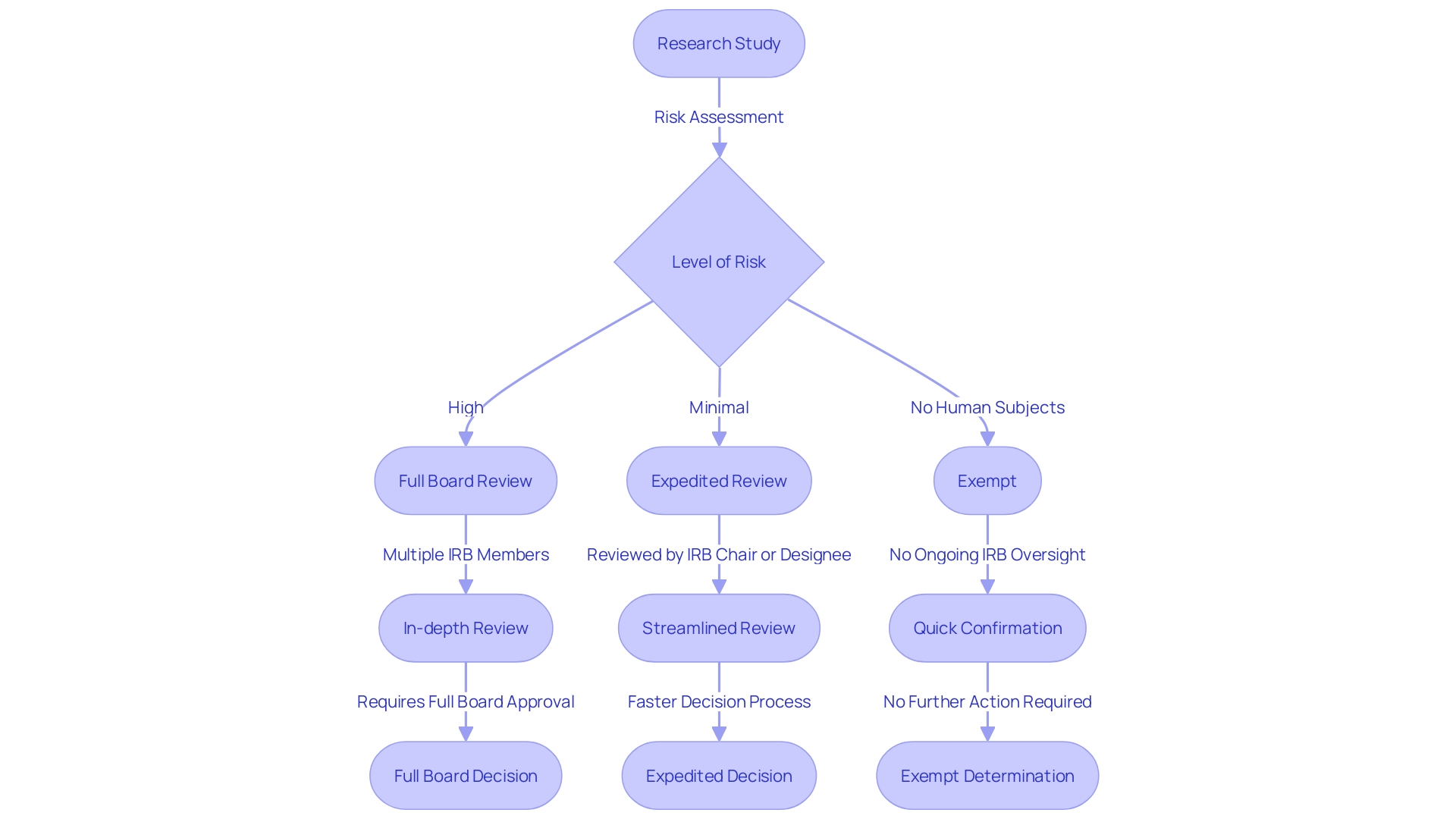
What Qualifies for Expedited Review
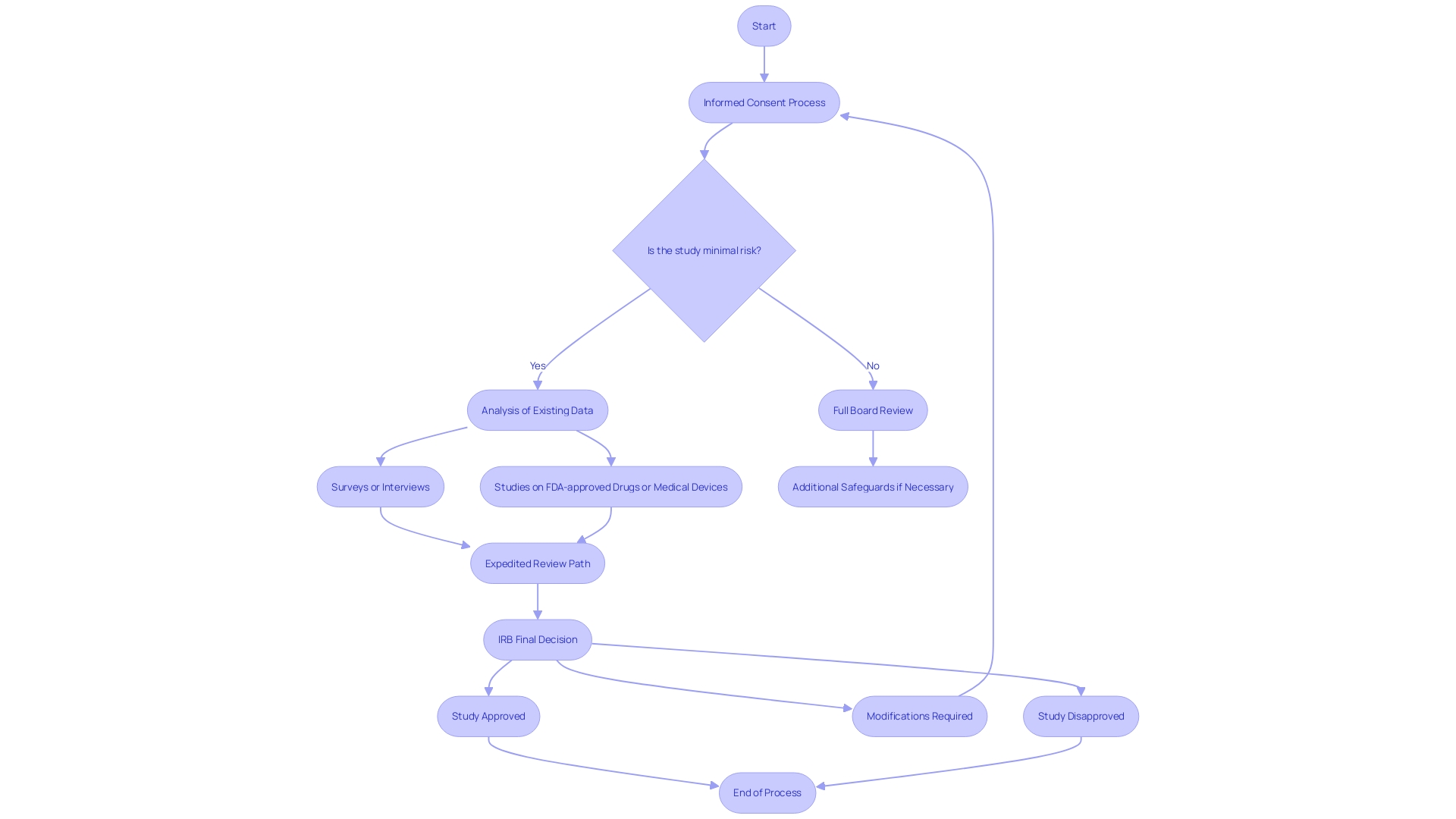
Accelerated evaluation processes by Institutional Review Boards (IRBs) are intended to streamline the assessment of studies that present minimal risk to participants. Such studies often involve analysis of existing data or biological specimens, as well as surveys or interviews that do not involve significant risk. Additionally, studies on drugs or medical devices that have received FDA approval or clearance may also qualify for an expedited review path. The FDA frequently employs qualitative methodologies, such as focus groups and in-depth interviews, to acquire nuanced insights into consumer perspectives on FDA-regulated products. This kind of investigation is crucial for creating variables for quantitative studies, comprehending attitudes and emotions, and exploring findings from quantitative inquiry. These qualitative studies, while not quantifiable in the traditional sense, are vital for informing policy decisions and for allocating resources effectively.
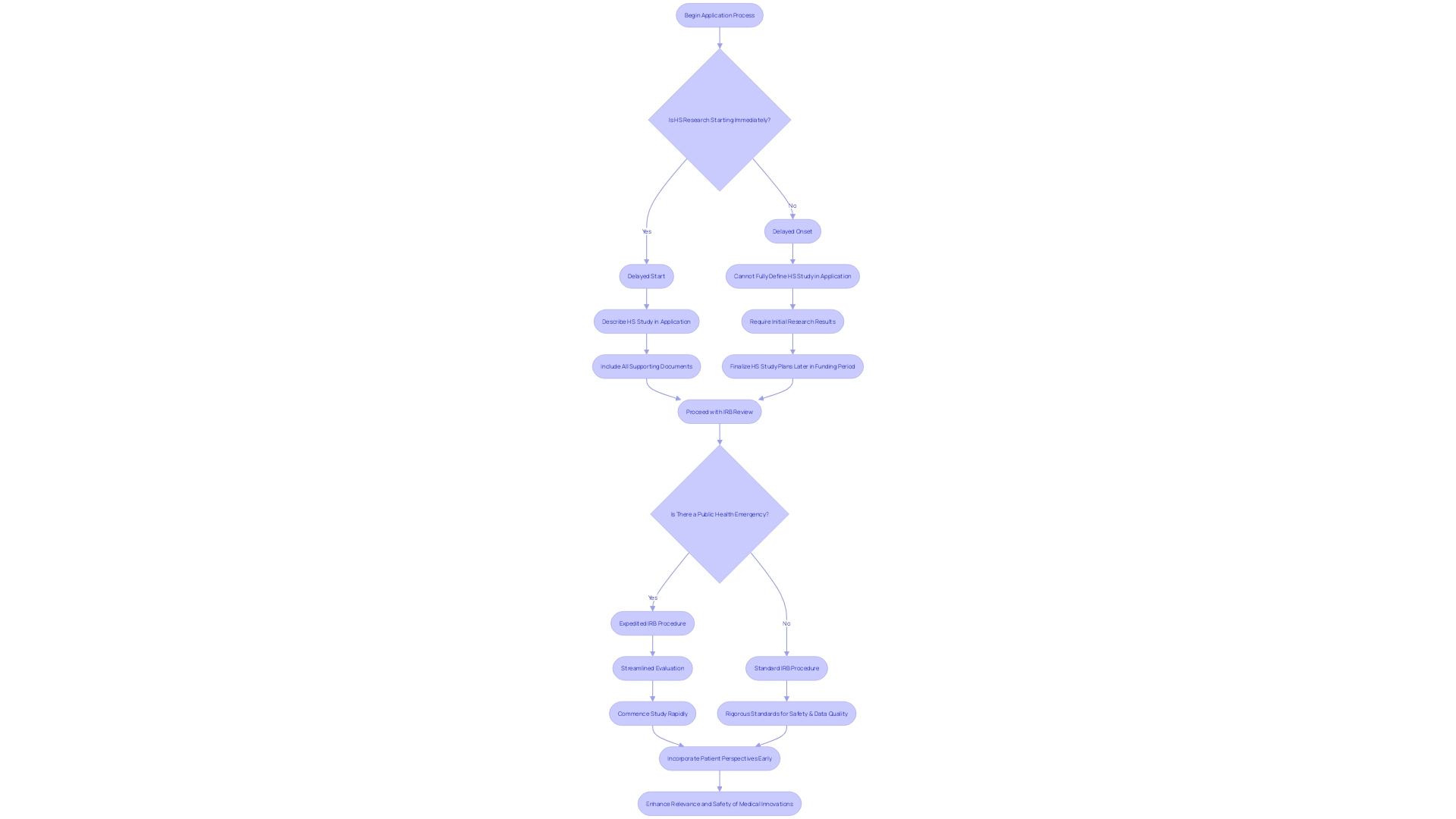
In the context of human subjects experimentation, it is crucial to distinguish between 'delayed start' and 'delayed onset' studies. A 'delayed start' study has a postponed initiation within the funding period but is fully defined in the application. In contrast, a 'delayed onset' study cannot be fully outlined upon application and requires initial investigation outcomes to finalize the human subjects study plans. Mistaking these terms can have significant implications on the application's evaluation or even result in a delay of award.
According to the latest evaluation standards, the FDA has consolidated five regulatory criteria into three key elements: the significance of the investigation, the thoroughness and viability of the study, and the proficiency and resources accessible. This framework highlights the influence, practicability, and scientific soundness of the investigation. Innovation also plays a critical role, whether through novel concepts, methods, or technologies, or by applying existing ones in unique ways to elevate the project's impact.
Benefits of Expedited Review
The accelerated Institutional Review Board (IRB) procedure is a crucial element in advancing medical studies, particularly when time is crucial. This streamlined approach allows for a more rapid commencement of studies, which is particularly vital in responding to urgent public health needs, such as the COVID-19 pandemic. With an average oncology trial today having 38 inclusion/exclusion criteria, and site-based clinical teams experiencing high turnover rates, the traditional full board evaluation can be a bottleneck, often taking weeks or months to finish. Expedited evaluation, on the other hand, ensures that essential studies can progress swiftly and with fewer administrative hurdles, all while maintaining rigorous standards for patient safety and data quality. The MHRA's pilot program exemplifies this commitment to efficiency, incorporating patient perspectives early on to enhance the relevance and safety of medical innovations. As research advances rapidly, with more than seven million academic papers published every year, the significance of a well-coordinated accelerated evaluation cannot be emphasized enough, providing hope to patients and contributing to the quality and reliability of the scientific undertaking.
Streamlining the IRB Process: Strategies and Innovations
To enhance the effectiveness of accelerated Institutional Review Board (IRB) procedures, it is crucial to embrace a versatile approach that involves the creation of transparent and accurate expedited assessment standards. This transparency ensures that researchers are well-equipped to prepare complete and precise Investigational New Drug (IND) applications. The incorporation of technological advancements plays a crucial part in enhancing the evaluation process. By adopting electronic submission systems and digital platforms, the management of documents is significantly simplified, and communication between IRBs and researchers is greatly improved.
Furthermore, fostering a collaborative environment between IRBs and researchers is paramount. Effective communication can expedite the resolution of any issues that surface during the evaluation, thus avoiding unnecessary delays. This is especially crucial in a scenario where secure and compliant investigation is of the utmost significance, particularly for projects that handle sensitive information and require government security clearances.
The FDA has acknowledged the significance of effective clinical investigation, and in reaction, has implemented various actions to improve the assessment procedure. These include efforts to harmonize human subject protection regulations with the Health and Human Services (HHS) Common Rule. This harmonization aims to increase the efficiency of clinical studies while safeguarding the rights of participants. Additionally, guidance on informed consent has been proposed to ensure clarity and aid participant understanding of the study's purpose, potential risks and benefits, and overall procedures.
In view of these developments, it is apparent that a coordinated endeavor to streamline the IRB evaluation procedure not only facilitates medical progress but also upholds the ethical standards necessary to protect research participants. By implementing these strategic approaches, the IRB can expedite without compromising the integrity or quality of the research.
The Role of Collaboration in Reducing Turnaround Time
Effective cooperation among researchers and Institutional Review Board (IRB) members greatly influences the speed at which expedited assessments are carried out. By sharing expertise and maintaining open lines of communication, all involved parties can ensure that the documentation and information required are provided promptly. Taking a proactive approach is crucial to prevent delays and expedite the review, which is particularly important in an era where up to 80% of clinical trials fail to meet their deadlines. Given the changing nature of biopharmaceutical development, where being the initial provider of new therapies can result in significant achievements, it becomes even more crucial to streamline these procedures. Collaboration not only promotes knowledge exchange but also allows for direct responses to queries or concerns that may arise, thereby improving the efficiency and quality of scholarly investigation. This is demonstrated by the transformative outcomes seen in interdisciplinary collaborations, where experts from diverse fields come together to solve complex issues, leading to innovations that are both medically effective and user-oriented. Furthermore, the incorporation of patient perspectives at the outset, as demonstrated by recent endeavors such as the MHRA Patient Engagement Approach, further illustrates the advantages of cooperative endeavors in influencing the course of clinical investigations.
Real-Time IRB Review: A Case Study
The idea of live IRB (Institutional Review Board) assessment is transforming the ethical supervision of clinical studies. This progressive model convenes researchers and IRB members for live discussions, either virtually or face-to-face, to address any questions or concerns on the spot. Such a dynamic approach eliminates the delays inherent in traditional correspondence, fostering immediate dialogue and collaboration. Real-time evaluation has the potential to significantly compress the assessment timeline, thereby accelerating the progress of vital research initiatives.
In the context of developing state-of-the-art medical devices like Pressure by IR-MED Inc., which is undergoing usability studies, the significance of prompt IRB procedures cannot be overstated. PressureSafe exemplifies the type of innovative healthcare technologies that benefit from expedited IRB reviews, ensuring that novel solutions reach patients in a timely manner while maintaining rigorous standards of safety and efficacy.
The procedure for conducting studies involving human subjects, especially when distinguishing between 'delayed start' and 'delayed onset' studies, is a crucial stage that gains advantages from the real-time IRB model. With the ability to discuss and resolve issues as they arise, IRB committees can help researchers navigate the complexities of their applications, leading to clearer and more accurate submissions.
Furthermore, the IRB's responsibility as the protector of ethical standards is crucial. Their evaluation system, which relies on experienced academics and experts, acts as the guardian for project proposals. The goal is always to ethically conduct investigations, minimize harm, and ensure participant consent. Real-time IRB assessments could improve this procedure, offering an immediate platform for dealing with any ethical dilemmas and strengthening the safeguarding of participants.
The incorporation of real-time IRB assessments can be viewed as a manifestation of the progress in medical research and technology. As healthcare continues to evolve, so too must the structures that support and regulate it, ensuring that innovation is not stifled by procedural delays while upholding the highest ethical standards.
Addressing Common Challenges in Expedited Reviews
Navigating the intricacies of expedited Institutional Review Board (IRB) processes for Investigational New Drug (IND) applications requires meticulous attention to detail. To be eligible for a fast-track evaluation, researchers must ensure that their study design fully complies with the IRB's specified criteria. This rigorous approach is crucial, as it aligns with the broader scientific community's commitment to upholding the integrity of investigations, something that has been profoundly underscored during critical health crises such as the COVID-19 pandemic.
The accelerated evaluation procedure requires ongoing adherence to the developing regulatory guidelines. Researchers are tasked with staying abreast of the latest updates to maintain the validity of their work. This dedication to adherence echoes recent alterations in regulatory assessment standards, emphasizing the importance, thoroughness, feasibility, and influence of investigation, as expressed by Dr. Lisa Steele and Dr. Stephanie Constant. Such criteria are redefining the evaluation of scientific endeavors, prioritizing projects with substantial potential impact and practical feasibility.
Additionally, the changing environment of scientific publication and peer evaluation has strengthened the need for precision and promptness in research dissemination. The increase in deceptive studies and the public's doubt towards scientific findings require an elevated level of examination in the peer review procedure. Therefore, researchers undertaking expedited IRB procedures must not only adhere to regulatory expectations but also responsibly contribute to the body of scientific knowledge, ensuring that their findings withstand the rigors of peer assessment.
In summary, while expedited IRB processes for IND applications offer a faster pathway to study approval, they come with the responsibility of meticulous planning, detailed alignment with IRB criteria, and a steadfast commitment to regulatory and ethical standards. This comprehensive approach safeguards the integrity of clinical studies and its consequential impact on public health.
Best Practices for Optimizing Expedited Review
Obtaining a fast-track assessment from an Institutional Review Board (IRB) for Investigational New Drug (IND) applications depends on a thorough comprehension of the IRB's particular expedited evaluation criteria and a well-organized study design. It is crucial to perform a thorough literature review to determine the originality and importance of your study within its relevant domain, which not only informs, but also simplifies the evaluation procedure. A distinct expression of the study objectives and questions, or hypothesis, is crucial, and must be prominently stated to facilitate the reviewer's assessment of the study's innovation and importance.
Proactive engagement with the IRB, characterized by transparency and intentional communication, is vital. By showcasing the community-led method for establishing optimal approaches in oceanographic studies, the incorporation of a wide range of knowledge and perspectives can greatly improve the effectiveness and availability of the results. By implementing this approach to the IRB, through guaranteeing that the application reflects a comprehension of the scientific community's requirements and the particular gaps the study intends to tackle, can promote a collaborative environment. This, coupled with detailing the rationale for the research and its place within the broader scientific context, can contribute to a more efficient and effective review process.
Conclusion
In conclusion, expedited review processes for Institutional Review Boards (IRBs) are crucial in protecting participants and upholding ethical standards in clinical research. There are three types of IRB review: full board, expedited, and exempt, each with its own criteria and level of scrutiny.
Expedited review is for studies that involve minimal risk and meet specific criteria, allowing researchers to move forward without a full board review. It offers a more rapid commencement of studies, particularly important for urgent public health needs. Strategies like transparent criteria, technological advancements, and collaboration between IRBs and researchers optimize the efficiency of the IRB process.
Real-time IRB review revolutionizes the ethical oversight of clinical research by fostering immediate dialogue and collaboration. It compresses the review timeline and ensures swift approval for innovative healthcare technologies.
Addressing common challenges in expedited reviews requires attention to detail, compliance with regulations, and a commitment to accuracy in research dissemination. Researchers must align their study designs with IRB criteria and contribute responsibly to scientific knowledge.
By implementing best practices and optimizing expedited review, researchers navigate the IRB process efficiently, ensuring research integrity and participant well-being. Expedited review and collaboration between researchers and IRBs advance medical research while upholding ethical standards.
Learn more about the benefits of expedited review for your clinical research studies.




