Overview
The article highlights best practices and expert insights for designing studies that secure regulatory approval within the medical sector. It asserts that a meticulously structured study design is crucial for demonstrating the safety and efficacy of medical devices and pharmaceuticals. By underscoring the necessity of adhering to regulatory standards and methodologies, the article enhances the likelihood of successful product approval. This focus on compliance not only facilitates smoother regulatory pathways but also builds trust in the research process, ultimately benefiting both developers and patients.
Introduction
In the dynamic world of clinical research, the design of a study transcends mere technicality; it stands as a pivotal determinant of success in securing regulatory approval. A well-structured study design forms the backbone of clinical trials, influencing every aspect from methodology to data collection and the interpretation of results.
With regulatory agencies such as the FDA and EMA demanding clarity and thoroughness, the necessity of crafting an effective study design has reached unprecedented levels of importance. This article delves into the multifaceted aspects of study design, examining its crucial role in navigating the regulatory landscape, optimizing clinical trials, and ultimately enhancing patient care through innovative medical devices.
By exploring various types of clinical studies and best practices that ensure compliance and success, this comprehensive overview offers invaluable insights for Medtech companies striving to excel in a competitive environment.
Understanding the Importance of Study Design in Regulatory Approval
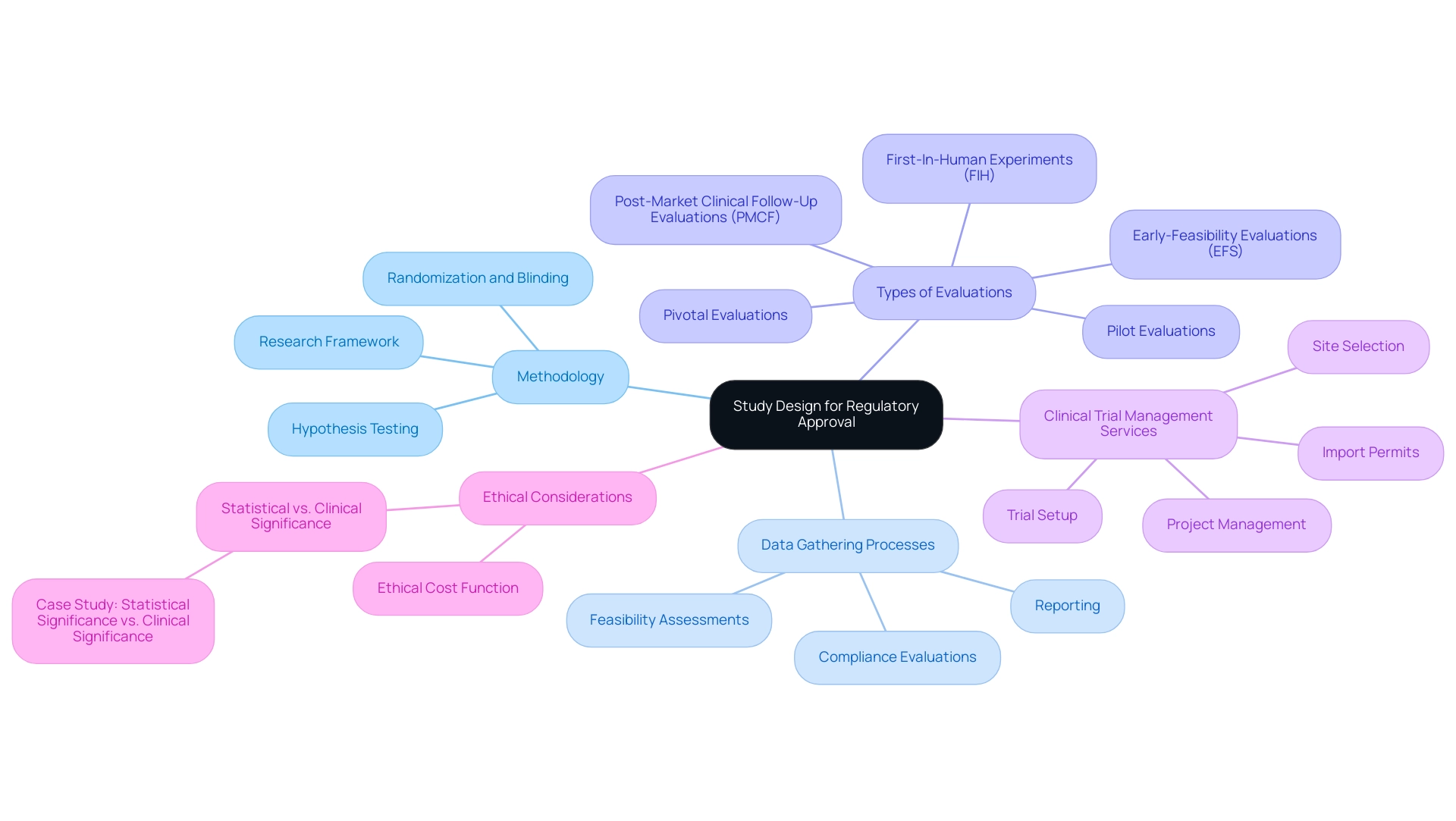
The study framework serves as the essential blueprint for any medical study, defining the methodology, data gathering processes, and analysis of results. A meticulously constructed study design for regulatory approval is crucial for obtaining regulatory authorization, as it guarantees that the examination effectively addresses the research question while upholding ethical and scientific standards. Regulatory organizations, including the FDA and EMA, mandate that the study design for regulatory approval be clear and comprehensive to thoroughly evaluate the safety and effectiveness of medical devices and pharmaceuticals.
At bioaccess®, we leverage over 20 years of expertise in overseeing experiments across Latin America, with a focus on Early-Feasibility Evaluations (EFS), First-In-Human Experiments (FIH), Pilot Evaluations, Pivotal Evaluations, and Post-Market Clinical Follow-Up Evaluations (PMCF). Our extensive clinical trial management services encompass:
- Feasibility assessments
- Site selection
- Compliance evaluations
- Trial setup
- Import permits
- Project management
- Reporting
Ensuring that every aspect of the research framework is meticulously planned and executed. Our customized approach enables us to tailor our services to meet the unique needs of each client.
An adequately powered research study ensures that the results are not only valid but also generalizable, a sentiment echoed by industry experts. This underscores the significance of research methodology in the medical device industry, where the implications of research results can profoundly impact patient care. A well-organized framework in the study design for regulatory approval not only minimizes biases but also enhances data integrity, thereby bolstering the credibility of the findings presented to regulatory bodies.
For instance, a study design for regulatory approval that incorporates randomization and blinding methods can significantly reduce bias, leading to more dependable results.
The importance of research methodology is further highlighted by the reality that larger-scale experiments are often employed to validate efficacy and safety evaluations through thorough hypothesis testing. Furthermore, innovative methods such as risk-based allocation frameworks enable higher-risk individuals to receive potentially superior therapies, addressing challenges associated with randomization in studies where patient involvement may be limited. Moreover, the ethical cost function in selection studies must be considered, as it evaluates the number of patients receiving suboptimal treatments and the severity of treatment errors.
This aspect is essential for understanding the ethical ramifications of research structure in medical experiments.
A case analysis illustrating the distinction between statistical significance and medical relevance emphasizes the critical role of the research framework in interpreting medical experiment outcomes, particularly concerning medical devices. Comprehending these concepts is vital for researchers and practitioners to make informed decisions about the relevance of experimental results to real-world situations.
In 2025, the emphasis on study design for regulatory approval remains paramount, especially regarding optimal methods for research project formulation. By adhering to established best practices, clinical trials can achieve higher success rates, ultimately facilitating the advancement of medical devices and enhancing patient outcomes. Katherine Ruiz, our specialist in Regulatory Affairs for medical devices and in vitro diagnostics in Colombia, plays a pivotal role in the study design for regulatory approval to ensure that our research designs meet all regulatory requirements.
Exploring Different Types of Clinical Studies for Regulatory Success
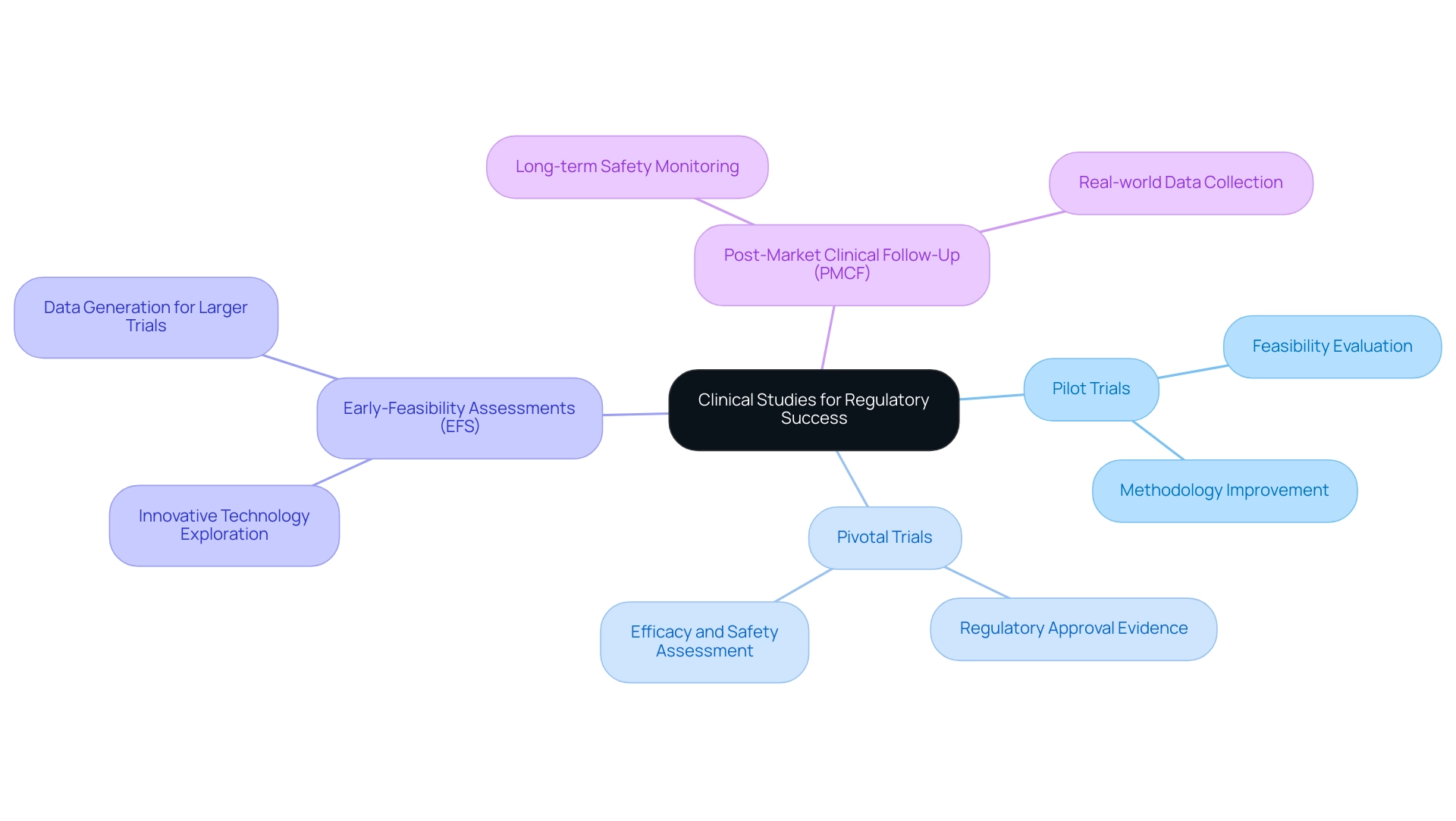
Clinical trials are integral to the study design for the regulatory approval process and can be categorized into several distinct types, each fulfilling a specific role.
- Pilot trials, often the initial phase in clinical research, are primarily conducted to evaluate the feasibility of a new medical device or technology. These investigations assist in recognizing potential challenges and improving methodologies before larger-scale trials commence.
- In contrast, pivotal research serves as a study design for regulatory approval, providing definitive evidence regarding a product's efficacy and safety, which is the cornerstone for regulatory submissions.
- Early-feasibility assessments (EFS) play a crucial role in exploring innovative technologies within a controlled environment. They generate critical data that can inform subsequent larger trials, ensuring that the development process is grounded in solid evidence. For instance, ReGelTec, Inc. successfully enrolled eleven patients in its Early Feasibility Study for HYDRAFIL™ in Barranquilla, Colombia, demonstrating the practical application of EFS in real-world settings.
- Additionally, post-market clinical follow-up evaluations (PMCF) are essential for monitoring the long-term safety and effectiveness of medical devices once they have received regulatory approval. These investigations help collect real-world data, which is vital for ongoing compliance and product enhancement.
Grasping the nuances of these research types is crucial for Medtech firms, such as bioaccess, seeking to enhance their study design for regulatory approval. With more than 20 years of experience in Medtech, bioaccess specializes in managing various research projects, including EFS, Pilot Trials, Pivotal Trials, and PMCF. For example, the success rates of preliminary experiments compared to pivotal investigations can differ considerably, with preliminary experiments frequently acting as a stepping stone to more extensive assessments.
Recent statistics suggest that incorporating advanced technologies, such as artificial intelligence and machine learning, can lower trial expenses by up to 20%, further improving the efficiency of research designs.
Expert opinions emphasize the significance of choosing the suitable research type. A senior director within a global biopharma's research sciences group noted that streamlining processes can save substantial time, stating, "Eliminating one 20-minute task per visit across 130,000 visits avoids 43,000 hours of work. Cras can focus on what matters."
This underscores the need for Medtech companies to not only select the appropriate study design for regulatory approval but also to optimize their operational processes.
As the landscape of clinical research evolves, there is a noticeable trend towards insourcing data management processes. This shift allows sponsors to maintain greater control over their data, leading to improved operational efficiency and higher quality outcomes. However, the reliance on spreadsheets for metadata management has proven challenging for companies looking to scale their operations effectively.
By understanding the distinct roles of pilot trials, pivotal trials, EFS, and PMCF, Medtech companies like bioaccess can strategically navigate the regulatory landscape with a focus on study design for regulatory approval, ultimately enhancing their chances of successful product approval.
A pertinent case analysis titled 'Shifting Towards In-House Data Management' illustrates this trend, showing how organizations that bring research in-house can ensure higher quality data management and better outcomes for patients. By leveraging these insights and adapting to the evolving data management landscape, Medtech companies can enhance their regulatory strategies and improve their chances of success.
Navigating Regulatory Requirements: Key Considerations for Study Design
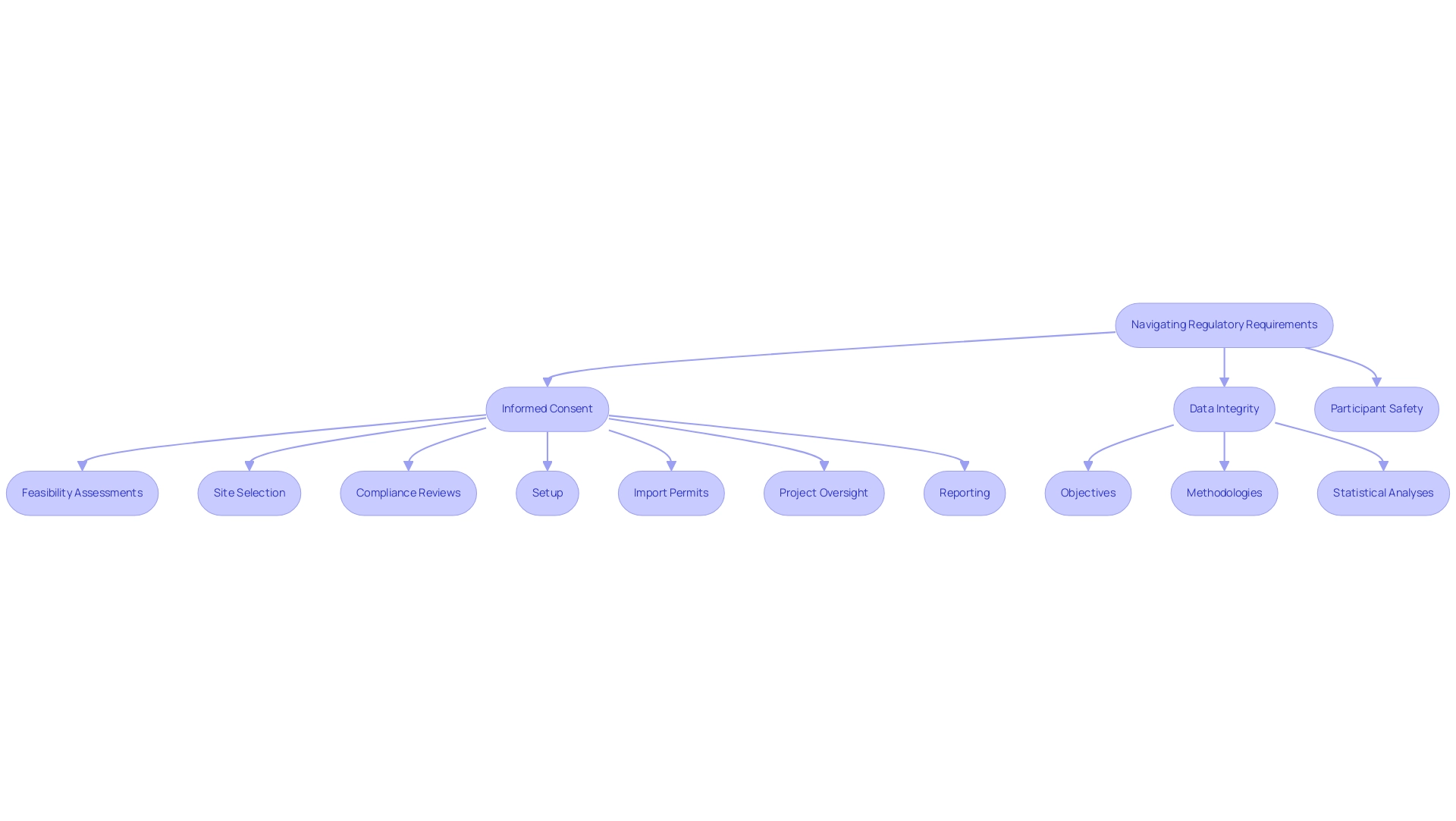
Navigating the regulatory landscape in Latin America necessitates a comprehensive understanding of the guidelines established by authorities such as the FDA and ICH. A crucial element of this process is ensuring that the study design for regulatory approval complies with Good Clinical Practice (GCP) standards, which highlight the ethical and scientific integrity of medical investigations. Essential components of a robust study design for regulatory approval include:
- Informed consent
- Data integrity
- Participant safety
These are critical factors for regulatory compliance.
As a prominent contract research organization, bioaccess® provides extensive management services for research that encompass:
- Feasibility assessments
- Site selection
- Compliance reviews
- Setup
- Import permits
- Project oversight
- Reporting
This holistic approach is crucial for navigating the complexities of the Latin American Medtech landscape, where understanding local regulations and market dynamics can significantly impact the success of clinical trials.
Regulatory bodies mandate meticulous documentation of the study design for regulatory approval, which includes:
- Objectives
- Methodologies
- Statistical analyses
For instance, a hazard ratio of 0.74 indicates that an evaluable sample size of 513 patients is necessary to achieve statistically significant results. This emphasizes the significance of meticulous planning during the planning stage to meet regulatory expectations.
Moreover, the study design for regulatory approval must align with both FDA and ICH guidelines, ensuring that all aspects of the research are compliant with current standards. Recent statistics suggest that adherence rates with GCP standards in studies have improved, yet challenges persist. Expert views highlight that it is the collective duty of lead researchers and statisticians to guarantee that study results are published, irrespective of their outcomes.
As Katherine Ruiz, a specialist in Regulatory Affairs for medical devices and in vitro diagnostics in Colombia, states, "It is an important responsibility of both the principal investigator and the statistician that the outcomes of trials are published, regardless of what those results are."
Case examples demonstrate the significance of efficient communication and teamwork between statisticians and principal investigators during the planning, analysis, and publication stages of research. One such case analysis emphasizes the importance of this collaboration, reinforcing that by proactively addressing regulatory requirements during the study design for regulatory approval, researchers can streamline the approval process and mitigate potential compliance issues, ultimately facilitating the advancement of medical devices in the market.
Best Practices for Designing Effective Clinical Studies
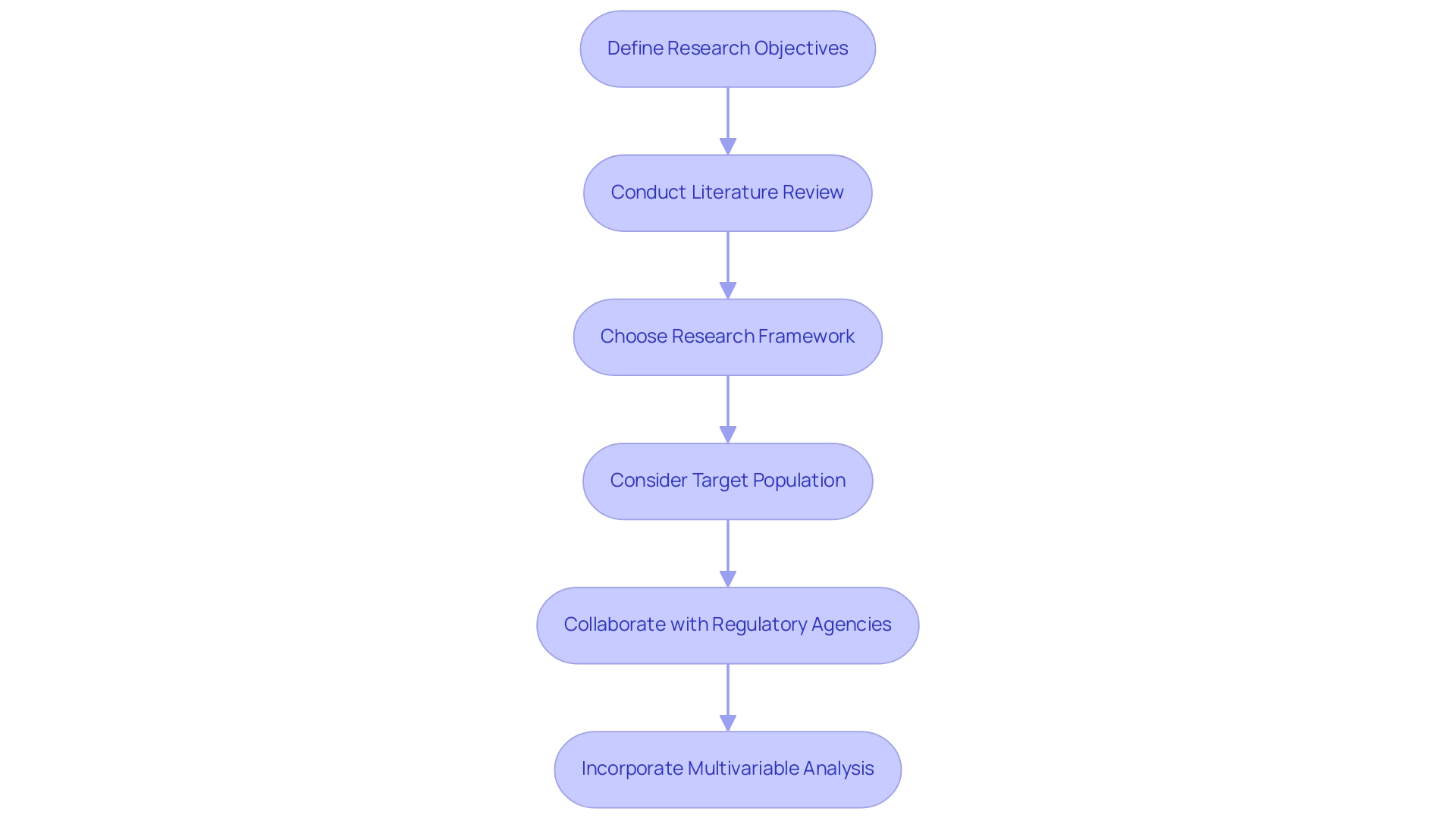
Creating effective clinical trials necessitates adherence to several best practices in study design for regulatory approval that can significantly enhance the likelihood of success. Researchers must first and foremost clearly define their research objectives, ensuring they are specific, measurable, achievable, relevant, and time-bound (SMART). This structured approach not only clarifies the goals but also facilitates the evaluation of outcomes.
Carrying out a comprehensive literature review is crucial to guide the research framework and pinpoint existing gaps in knowledge. This step allows researchers to build upon previous findings and avoid redundancy, ultimately contributing to the advancement of medical knowledge.
Choosing a suitable research framework is essential and must correspond with the inquiry in question. Options may consist of:
- Randomized controlled trials (RCTs)
- Observational research
- Adaptive approaches
Each providing distinct benefits based on the context of the investigation. For instance, RCTs are frequently regarded as the gold standard for establishing causality, while adaptive approaches permit modifications based on interim results, enhancing flexibility and efficiency.
Careful consideration of the target population is also vital. Researchers should ensure that inclusion and exclusion criteria are well-defined, which not only enhances participant recruitment and retention but also improves the generalizability of the findings. A well-defined population can lead to more robust data and ultimately more reliable conclusions.
Collaborating with regulatory agencies early in the research planning process is another best practice that can lead to a more effective study design for regulatory approval and provide substantial advantages. By soliciting feedback from these entities, researchers can identify potential issues before they arise, which helps in developing a study design for regulatory approval that streamlines the process and reduces the risk of delays. In Latin America, for instance, understanding the role of INVIMA, the Colombia National Food and Drug Surveillance Institute, is crucial for compliance and oversight in medical device trials.
Incorporating multivariable analysis into the research design can further enhance the validity of research findings. This approach enables the examination of several inter-correlated variables at once, offering a more comprehensive understanding of complex medical data. Research has demonstrated that employing multivariable techniques can result in more refined insights, thus enhancing the overall quality of medical investigations.
As emphasized in a recent case examination, multivariable analysis offers a more thorough understanding of intricate medical data, improving the validity of research results. Additionally, the use of statistical methods, such as Kaplan-Meier survival curves, can depict survival time as time to the final effect, such as tumor recurrence or mortality, illustrating the importance of analytical tools in medical research.
As Dr. Dave Nicholas noted, "The authors acknowledge the sincere efforts of Dr. Dave Nicholas in reviewing and developing the manuscript," emphasizing the importance of expert review in research study design.
By applying these best practices, including well-crafted protocols, impartial subject selection, and suitable analytical tools, researchers can improve their study design for regulatory approval. With bioaccess®'s 20+ years of experience in Medtech and expertise in managing Early-Feasibility, First-In-Human, Pilot, Pivotal, and Post-Market Follow-Up Studies, along with a customized approach to clinical study management, the successful advancement of medical devices and improved patient outcomes can be achieved.
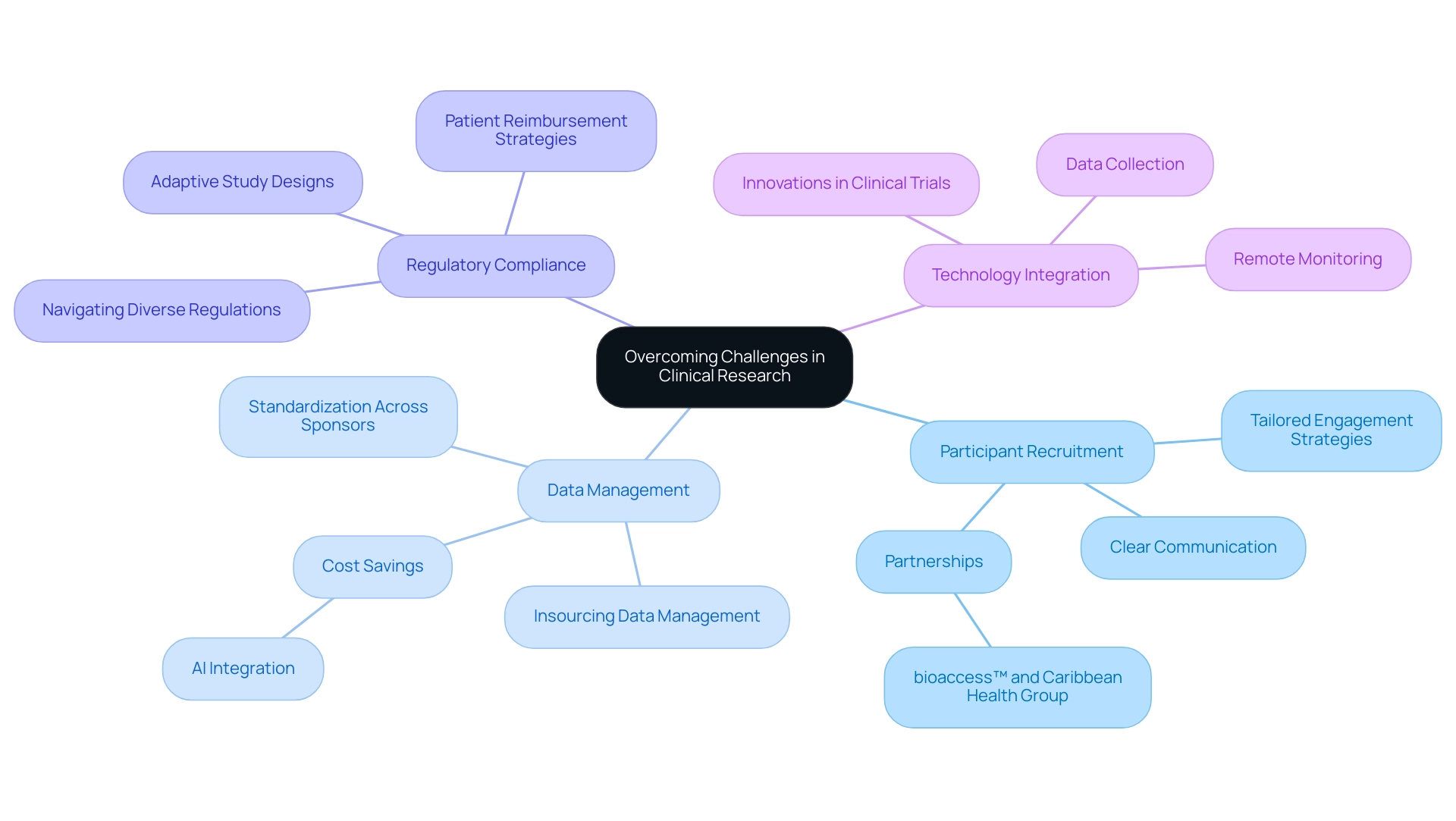
Overcoming Challenges in Clinical Research Through Strategic Study Design
Clinical research frequently encounters significant challenges, particularly in participant recruitment, retention, and adherence to regulatory requirements. A strategic approach to study design for regulatory approval is essential for effectively navigating these obstacles. For instance, adaptive study designs offer enhanced flexibility, enabling researchers to make real-time adjustments based on interim results, which can lead to more efficient processes and improved outcomes.
In 2025, the trend towards insourcing data management is transforming how sponsors approach clinical studies. By owning their data, sponsors can streamline processes and reduce reliance on contract research organizations (CROs), potentially achieving cost savings of up to 20% through the integration of AI and machine learning technologies. Bree Burks, Vice President of Site Strategy at Veeva, observes that as sponsors reconsider their site engagement approaches, they will emphasize consistent site technology and standardization across sponsors for all studies. This shift not only enhances data integrity but also allows for more tailored participant engagement strategies.
Moreover, leveraging technology for remote monitoring and data collection has proven effective in boosting participant engagement and retention rates. Clear communication of study objectives and expectations is crucial; it fosters trust and commitment among participants, which is essential for successful execution.
A significant partnership between bioaccess™ and Caribbean Health Group seeks to establish Barranquilla as a primary location for clinical research in Latin America, backed by Colombia's Minister of Health. This partnership is designed to enhance the feasibility and selection of research sites, compliance reviews, trial setup, project management, and reporting, ultimately contributing to local economic growth through job creation and healthcare improvement.
Additionally, Katherine Ruiz's expertise in regulatory affairs for medical devices and in vitro diagnostics in Colombia plays a crucial role in ensuring that documentation complies with country requirements, further strengthening the collaboration's impact.
A case study titled "Challenges of Differing Regulations in Global Trials" illustrates the complexities sponsors face. The growing complexity of global clinical studies necessitates navigating diverse regulatory environments, complicating patient enrollment and retention. Regulatory disparities and the need for patient reimbursement strategies are recognized as significant obstacles, complicating the execution of global studies. This highlights the necessity for strategic planning in study design for regulatory approval that anticipates these challenges and incorporates solutions to facilitate smoother execution.
By proactively addressing potential challenges and integrating innovative solutions into the research framework, investigators can significantly enhance the likelihood of successful execution through a robust study design for regulatory approval, ultimately advancing the development of medical devices that can improve lives. Moreover, collaborations such as that of bioaccess™ with GlobalCare Clinical Studies have shown impressive outcomes, attaining over a 50% decrease in recruitment duration and 95% retention rates, underscoring the influence of efficient research management services.
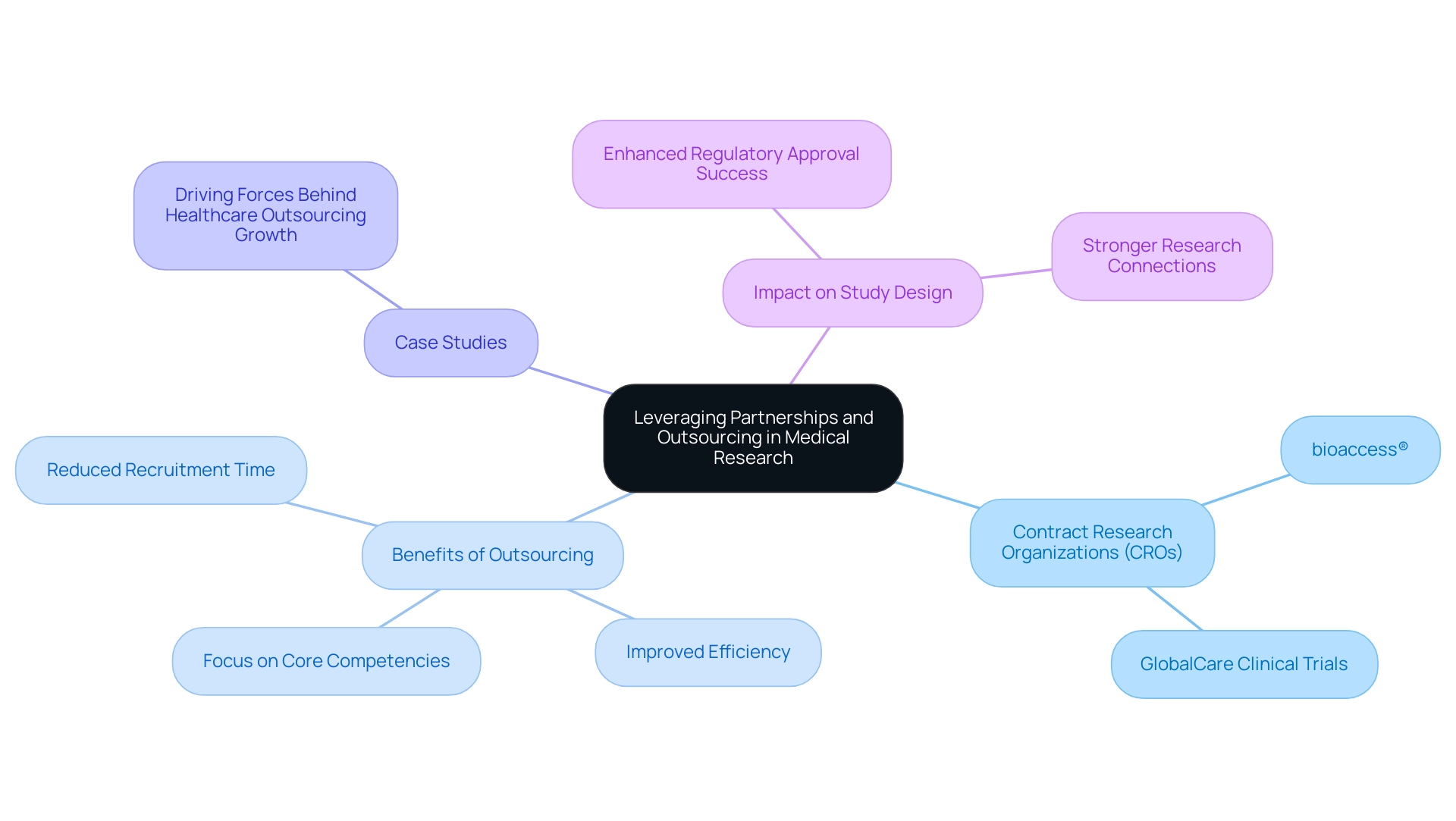
Leveraging Partnerships and Outsourcing for Successful Study Design
In the intricate realm of medical research, the strategic use of partnerships and outsourcing emerges as a pivotal factor in enhancing study design and execution. Collaborating with contract research organizations (CROs) such as bioaccess® equips Medtech companies with specialized expertise, essential resources, and invaluable local insights that can significantly streamline the research process. Notably, Dushyanth Surakanti, Founder and CEO of Sparta Biomedical, shared his positive experience with bioaccess® during its initial human testing in Colombia, underscoring the effectiveness of such collaborations.
Building strong connections with researchers, regulatory agencies, and ethics boards is essential for promoting effective dialogue and cooperation, which are crucial for managing the intricacies of research studies. The partnership between bioaccess™ and Caribbean Health Group aims to establish Barranquilla as a prominent location for research in Latin America—a vision endorsed by Colombia's Minister of Health, Juan Pablo Uribe. This initiative not only enhances the local research landscape but also attracts more trial projects to the region.
Outsourcing specific functions—such as data management, patient recruitment, and regulatory submissions—enables research teams to concentrate on their core activities while ensuring that specialized tasks are managed by experts. This approach not only improves operational efficiency but also allows for a more agile response to the dynamic demands of research. For instance, GlobalCare Clinical Trials' partnership with bioaccess™ has resulted in an impressive over 50% reduction in recruitment time and a remarkable 95% retention rate, demonstrating the tangible benefits of effective collaboration.
The advantages of outsourcing within the research framework are underscored by recent data indicating that over 30% of the global Full-Time Equivalent workload is projected to be managed by CRO partners. This trend reflects a growing recognition of the benefits that outsourcing offers, including improved focus on core competencies and enhanced project timelines.
Successful case studies illustrate how leveraging partnerships in research can lead to superior study design for regulatory approval and higher success rates. Organizations that have effectively integrated CRO partnerships into their operational strategies report increased efficiency and better alignment with study designs for regulatory approval. The driving forces behind healthcare outsourcing growth are reshaping the industry, providing opportunities for healthcare companies to enhance their operations and concentrate on core competencies.
Moreover, as Maria De Jesus aptly points out, "Healthcare workers are stretched thin. Long shifts, endless paperwork, and nonstop patient demands leave little room for handling patient care services phone calls." This highlights the importance of efficient communication, further emphasizing the need for outsourcing and partnerships in clinical research.
In summary, by strategically leveraging partnerships and outsourcing, Medtech companies can optimize their design processes and improve their chances of achieving regulatory success through a study design for regulatory approval, ultimately advancing the development of innovative medical devices that can transform patient care. Choosing CRO partners with proven track records, like bioaccess®, is essential for fostering strong and efficient relationships that can meet project timelines and enhance overall study outcomes.
Conclusion
Effective study design is paramount in clinical research, profoundly impacting regulatory approval and patient care. A meticulously structured study forms the backbone of clinical trials, directing methodology and data collection while adhering to the stringent standards established by regulatory agencies such as the FDA and EMA. By employing diverse study types—including Early-Feasibility Studies and Post-Market Clinical Follow-Up Studies—Medtech companies can refine research processes and bolster regulatory submissions.
Grasping Good Clinical Practice (GCP) standards is critical for maneuvering through the regulatory landscape. Adopting best practices—such as formulating SMART objectives and engaging with regulatory bodies from the outset—significantly boosts the likelihood of success. Moreover, adaptive trial designs and cutting-edge data management technologies address challenges related to participant recruitment and retention.
Strategic partnerships and outsourcing play a crucial role, enabling Medtech companies to tap into specialized expertise and resources that enhance operational efficiency. Successful collaborations have demonstrated substantial benefits, including expedited recruitment times and heightened retention rates.
In conclusion, prioritizing effective study design is vital for regulatory compliance and the progression of medical innovation. By embracing these principles and nurturing collaborative relationships, Medtech companies can improve their prospects for regulatory success and contribute to enhanced patient outcomes through innovative medical devices. This focus is essential for the future of clinical research.
Frequently Asked Questions
What is the purpose of a study framework in medical research?
The study framework serves as the essential blueprint for any medical study, defining the methodology, data gathering processes, and analysis of results. It ensures that the examination effectively addresses the research question while upholding ethical and scientific standards.
Why is a meticulously constructed study design important for regulatory approval?
A meticulously constructed study design is crucial for obtaining regulatory authorization as it guarantees that the research adheres to ethical and scientific standards, allowing regulatory organizations to thoroughly evaluate the safety and effectiveness of medical devices and pharmaceuticals.
What types of clinical trials are included in the study design for regulatory approval?
The study design for regulatory approval includes various types of clinical trials such as Pilot Trials, Pivotal Trials, Early-Feasibility Evaluations (EFS), and Post-Market Clinical Follow-Up Evaluations (PMCF).
What is the role of Pilot Trials in clinical research?
Pilot Trials are primarily conducted to evaluate the feasibility of a new medical device or technology, helping to identify potential challenges and improve methodologies before larger-scale trials commence.
How do Pivotal Trials contribute to regulatory submissions?
Pivotal Trials provide definitive evidence regarding a product's efficacy and safety, which is essential for regulatory submissions and approval processes.
What is the significance of Early-Feasibility Evaluations (EFS)?
EFS plays a crucial role in exploring innovative technologies within a controlled environment, generating critical data that informs subsequent larger trials, ensuring the development process is grounded in solid evidence.
What are Post-Market Clinical Follow-Up Evaluations (PMCF) used for?
PMCF are essential for monitoring the long-term safety and effectiveness of medical devices after they have received regulatory approval, collecting real-world data vital for ongoing compliance and product enhancement.
How does a well-organized study design minimize biases?
A well-organized framework in the study design incorporates methods such as randomization and blinding, which significantly reduce bias and enhance data integrity, bolstering the credibility of findings presented to regulatory bodies.
What is the impact of research methodology on patient care in the medical device industry?
Research methodology is significant as it ensures that results are valid and generalizable, ultimately impacting patient care by informing decisions related to medical devices and treatments.
How can advanced technologies improve clinical trials?
Incorporating advanced technologies, such as artificial intelligence and machine learning, can lower trial expenses by up to 20%, enhancing the efficiency of research designs and potentially increasing success rates.
What trend is observed in data management processes within clinical research?
There is a noticeable trend towards insourcing data management processes, allowing sponsors to maintain greater control over their data, leading to improved operational efficiency and higher quality outcomes.

