Introduction
Clinical trial consulting services have a significant impact on the efficiency and effectiveness of clinical trial conduct. These services provide expertise in strategic planning and decision-making, ultimately improving the quality of research.
Furthermore, they play a crucial role in the patient experience, offering support in areas such as logistics and travel coordination. This article explores the benefits of clinical trial consulting services, including streamlined study design, efficient site selection, enhanced regulatory compliance, and optimized resource utilization. Through this examination, it becomes evident that these services are indispensable in ensuring successful and impactful clinical studies.
Benefits of Clinical Trial Consulting Services
Delving into the realm of clinical research, the auxiliary services of clinical trial consulting boast a major impact on the efficiency and efficacy of trial conduct. These services instigate stronger foundations for studies by fortifying decision-making processes. As reflected in years of transaction advisory expertise noted by industry professionals, companies often retrospectively acknowledge that their methodologies for Phase I-III studies could have been more robust.
Analysis of 1,200 data points suggests that around 80% of critical decisions made early in the research could be markedly improved with thorough strategic planning and proactive advisement. The patient experience further underscores the value of consulting services. When a patient from rural Pennsylvania with an ultra-rare illness is chosen for a clinical trial in Turkey, the logistics become daunting.
Securing visas, navigating foreign documentation, and organizing intricate travel plans are just the tip of the iceberg. This scenario exemplifies the need for strategic oversight that encompasses patient-centric considerations, ensuring a seamless journey from recruitment to the completion of the trial. It's in these intricate navigations that clinical trial consulting services demonstrate their indispensable role, streamlining both preparatory decisions and patient experience for a cohesive research endeavor.
Streamlined Study Design
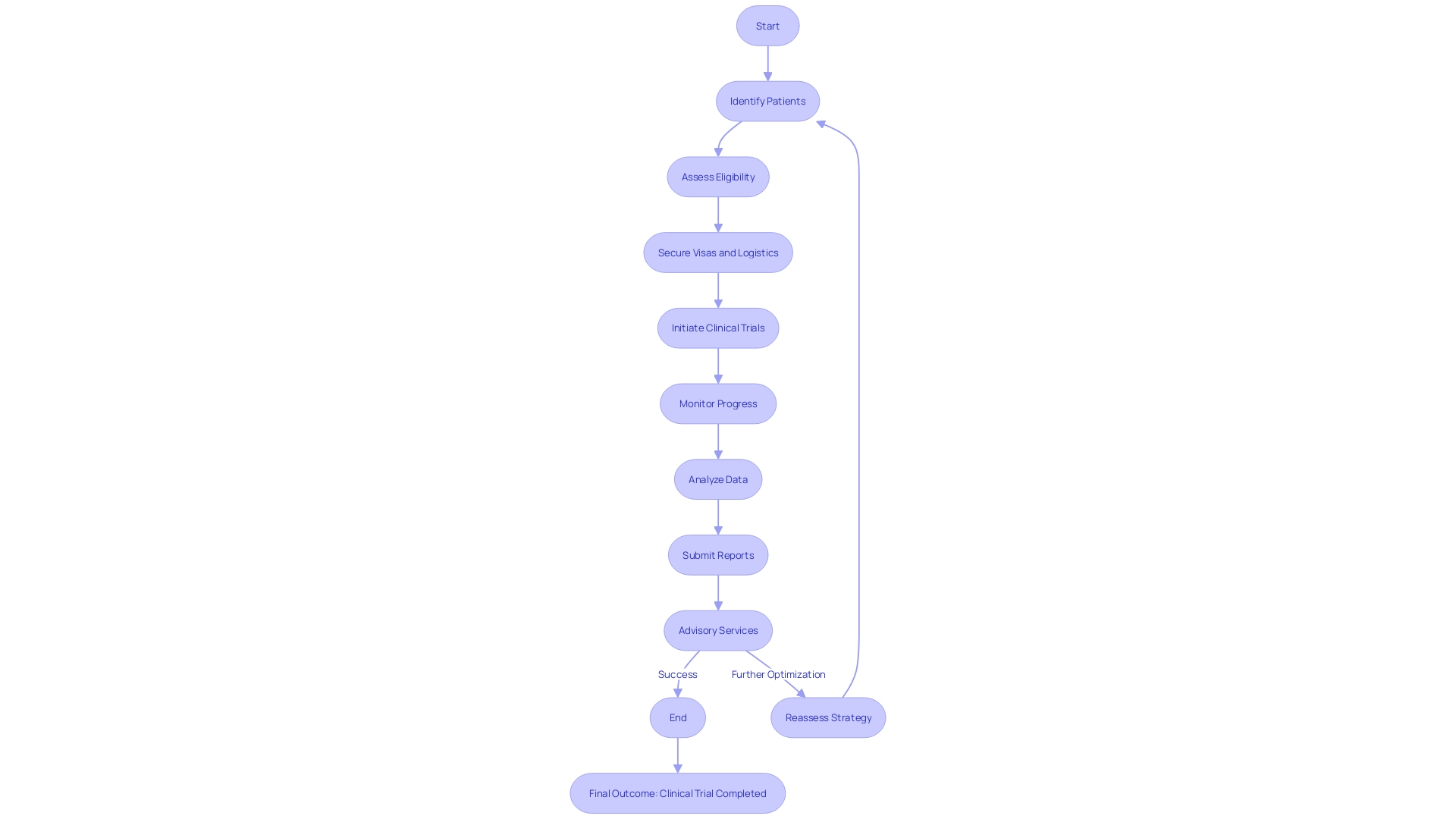
Clinical trial consulting services play an indispensable role in orchestrating the complexities of study design, a task that involves a harmonious blend of scientific precision and thoughtful consideration of ethical, legal, and social implications. They provide researchers with robust expertise in protocol development to create highly structured and scientifically valid studies.
This guidance includes key design aspects such as the meticulous determination of inclusion and exclusion criteria, the implementation of randomization techniques, and the formulation of data collection strategies. Particularly in the governance of rapidly evolving medical technologies, consulting services take a holistic approach, considering a broad array of elements that influence the progress of emerging inventions.
They draw from case studies that explore success and challenge in sector-spanning governance, accompanied by illustrative vignettes that underscore the specific ethical quandaries faced in today's medical technology landscape. Such services become even more crucial when factoring in the complexities of international clinical trials, as illustrated by the plight of a patient in rural Pennsylvania navigating the intricate process of participating in a trial abroad. This vignette lays bare the logistical hurdles faced, such as securing a visa, managing foreign documents, and coordinating travel. Through their comprehensive approach, clinical trial consulting services provide a pivotal support system that aims to streamline these issues, facilitating the successful execution of trials that contribute to medical advances while also addressing the critical human factors at play.
Efficient Site Selection
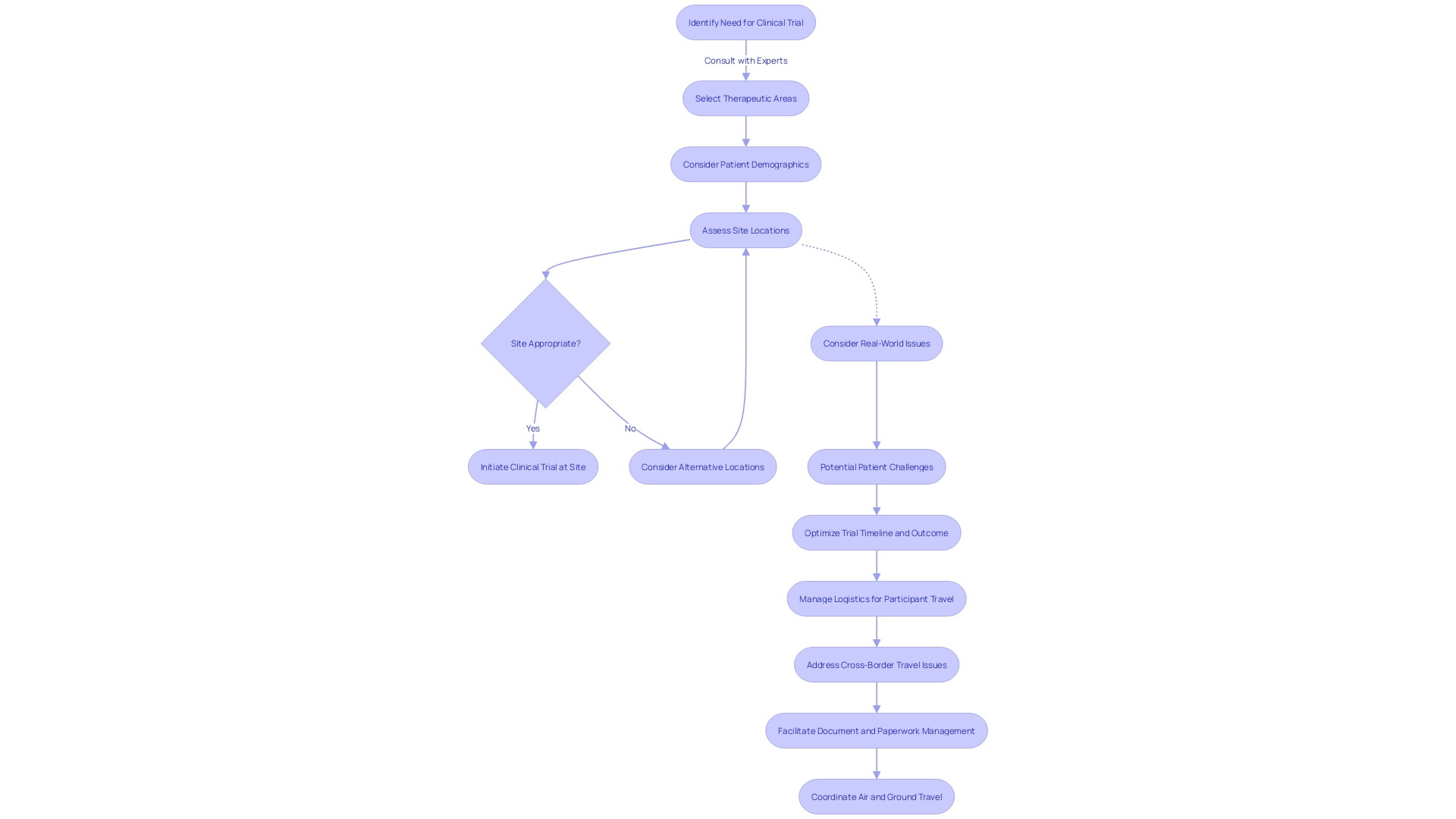
In the intricate landscape of clinical trials, the selection of appropriate sites is pivotal in driving the success of a research study. Consulting experts are integral in navigating this complex process, bringing to bear their extensive industry knowledge and a well-established network.
These professionals aptly discern the optimal locations for trials, taking into account the specific therapeutic areas, target patient demographics, and infrastructural prerequisites. The insights offered by these consultants are informed by real-world issues highlighted in recent discussions at the JAMA Summit.
For instance, with over 40,000 registered RCTs annually, discrepancies between trial designs and clinical applications can lead to inefficiencies and reduced impact on medical guidelines. This insight strengthens the consultant's ability to tailor site selection to not only facilitate swift patient recruitment and streamline the execution of the trial but also to bridge the gap between the rigorous control of clinical trials and the diverse conditions of clinical practice. Such precision in site selection can prevent logistical burdens that patients may face, like those of a patient from rural Pennsylvania who must negotiate international travel to participate in a trial in Turkey, grappling with obtaining visas, overcoming language barriers, and coordinating transport. Thus, clinical trial consultants play an indispensable role in enhancing the trial process and mitigating potential challenges, with a clear focus on optimizing the timeline and outcome of clinical studies.
Enhanced Regulatory Compliance
Clinical trial companies are essential operators within the biopharmaceutical industry, providing not just expertise but also ensuring the safety and efficacy of new medications and interventions through rigorously conducted studies. These businesses are imperative in the execution of clinical trials, which include the testing of new treatments such as drugs, behavioral interventions, and devices.
To maintain public trust and advance medical innovation, they adhere to stringent standards, navigating complex global regulatory frameworks and integrating emerging technologies like artificial intelligence (AI) and machine learning (ML). For example, Japan's first and largest Contract Research Organization (CRO), CMIC Group, demonstrates how comprehensive services spanning from drug development to market entry solutions can tailor to different phases of a treatment's lifecycle.
They exemplify the industry’s dedication to meeting diverse customer needs and remaining at the forefront of innovation. The global nature of clinical research adds another layer of intricacy, as patients from different regions, such as a rural Pennsylvania suffering from a rare disease, might have to travel internationally to participate in trials.
These scenarios emphasize the significance of clinical trial companies in coordinating logistics and ensuring clear communication across borders, mitigating the stress of complex procedures for participants. Moreover, balancing patient safety with the bold strides of innovation is a delicate dance that clinical trial companies perform daily. Guidelines from international regulatory bodies, including the latest EU AI Act, demand a meticulous approach to integrating AI into clinical research, requiring transparent risk-based methods. The significance and responsibilities of clinical trial companies cannot be overstated, as they are pivotal in validating new treatments through different trial phases, each aimed at specific study objectives to establish safety and effectiveness, thereby influencing patient care and the future of healthcare.
Optimized Resource Utilization
Within the ambit of clinical trial management, consulting services are increasingly vital in addressing logistical intricacies and enhancing operational efficiency. For example, a patient from rural Pennsylvania with an ultra-rare illness may be invited to join a clinical trial abroad, such as in Turkey.
Despite the potential benefits, the patient faces the daunting task of navigating international travel logistics, from obtaining visas to handling unfamiliar paperwork, all of which could jeopardize their participation. Clinical trial consultants step in to preempt such bottlenecks, employing strategies to facilitate each aspect of resource deployment, from patient recruitment to data collection, ensuring no detail is overlooked.
They draw upon their expertise to refine research processes, integrating insights like those from a study on 'EHR-sourced' trials, which highlighted existing uncertainties in trial infrastructure and site capabilities. By meticulously planning and executing resource allocation, echoed in the insights from seasoned advisors at Tree hill Partners, over 80% of early-stage decisions could be significantly improved. Thus, consultants play an indispensable role in ensuring that trials not only adhere to budgetary limits but also uphold the integrity and feasibility of life-altering research ventures.
Conclusion
In conclusion, clinical trial consulting services are essential for the success of clinical studies. They provide strategic planning, streamline study design, and ensure efficient site selection.
These services also enhance regulatory compliance, balancing patient safety with innovation. Moreover, clinical trial consultants optimize resource utilization, addressing logistical intricacies and improving operational efficiency. Overall, these services play a vital role in advancing medical knowledge and shaping the future of healthcare.




