Introduction
The orchestration of clinical trials is a multifaceted endeavor, often laden with intricate challenges that span beyond the scientific and regulatory scope. From navigating legal and practical obstacles in international trials to streamlining patient recruitment and enrollment processes, the management of clinical trials requires a breadth of expertise. Contract Research Organizations (CROs) play a critical role in overcoming these challenges, providing end-to-end solutions and specialized services that enhance the efficiency and scope of clinical trial companies.
This article explores the benefits of outsourcing to CROs, their role in strategic site identification and activation, and the streamlined expertise and cost efficiency they offer. Additionally, it discusses the focus on core competencies and risk mitigation, as well as the global reach and regulatory compliance provided by CROs. By understanding the pivotal role of CROs in clinical trial management, pharmaceutical entities can optimize their operations and advance patient care.
Challenges in Managing Clinical Trials
The orchestration of clinical trials is a multifaceted endeavor, often laden with intricate challenges that span beyond the scientific and regulatory scope. For instance, envision a patient from rural Pennsylvania grappling with an ultra-rare disease.
Despite the absence of FDA-approved treatments, hope emerges as they are presented with the chance to join a clinical trial in Turkey. The complexity of the trial is not just in its protocol, but also in the logistical hurdles that patients face.
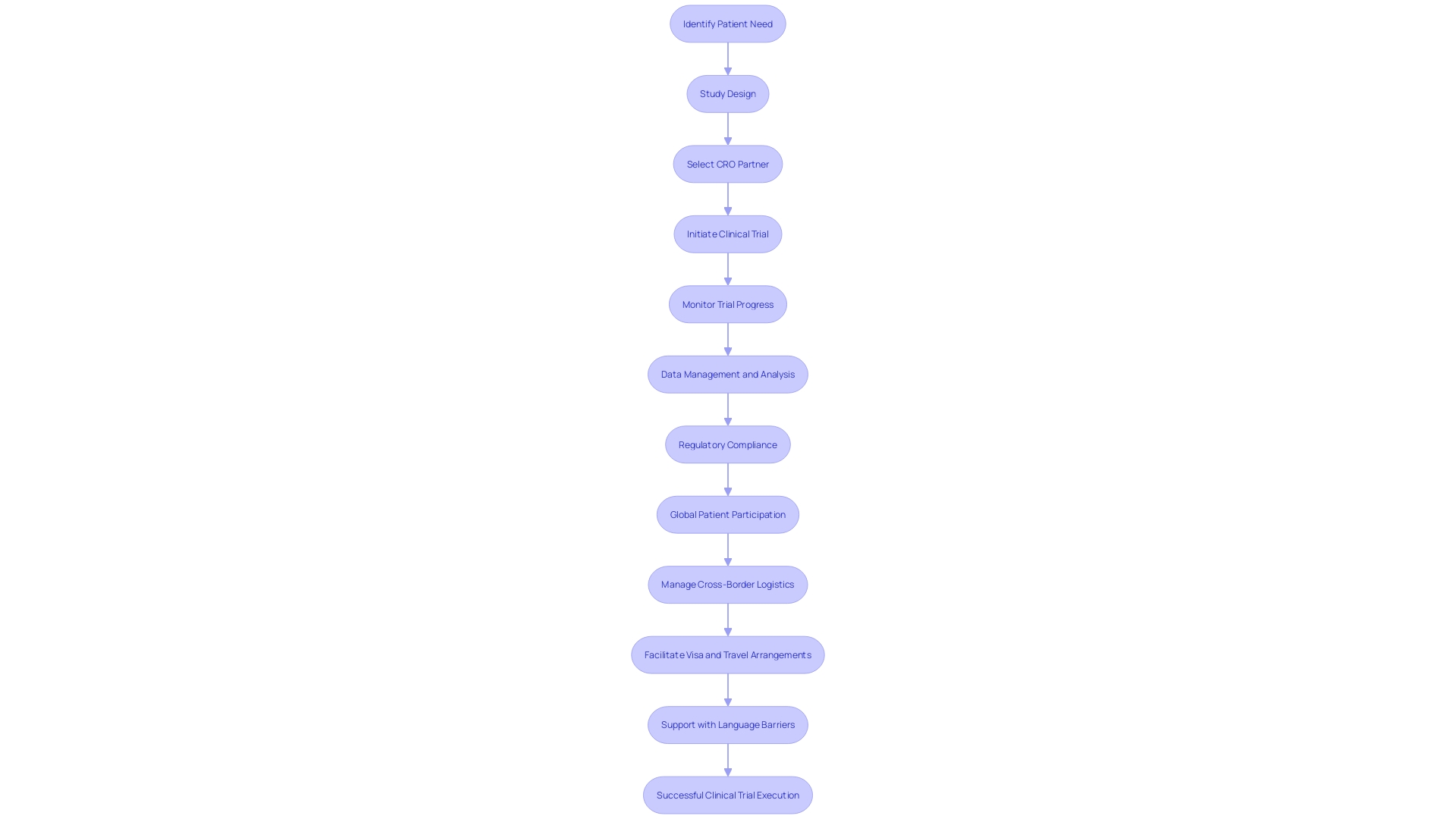
International trials like these require navigating a labyrinth of legal and practical challenges, from securing travel visas to deciphering foreign documentation, all while ensuring the patient's well-being. It's a testament to the breadth of expertise needed in clinical trial management—encompassing patient liaison and logistical coordination, on top of the core competencies in study design and data analysis. The real-world complexities underscore the escalating demands placed on clinical trial companies to execute studies efficiently, even when they span across continents and cultures.
The Role of CROs in Overcoming Challenges
Contract Research Organizations (CROs), such as CMIC Group which initiated the CRO industry in Japan, provide end-to-end solutions that are indispensable in tackling the multifaceted challenges of clinical trial management. These organizations bring to the table a wealth of expertise, robust infrastructure, and a proven track record of managing clinical trials with precision and efficiency.
CROss like CMIC offer a range of services that span the entire pharmaceutical value-chain, thereby enabling clinical trial companies to enhance their operational capabilities and resource utilization. They stand out not only for their ability to conduct and manage trials but also for their innovative approaches in addressing the logistical and regulatory complexities that trials may present.
For instance, a patient with a rare disease in rural Pennsylvania may face the daunting task of participating in a trial abroad, such as in Turkey. The logistical hurdles, including obtaining visas, managing foreign paperwork, and arranging travel, can be overwhelming. CROss step in to streamline these processes, ensuring that patients can focus on the trial without the added stress of cross-border challenges. Their tailored solutions extend to pharmaceutical companies, medical device manufacturers, academia, bio-ventures, and medical institutions, illustrating their critical role in advancing clinical research and patient care.
Benefits of Outsourcing to CROs
Clinical Research Organizations (CROs) are pivotal in enhancing the scope and efficiency of clinical trial companies by providing a multitude of specialized services. An illustrative case is the scenario of a patient from rural Pennsylvania with an ultra-rare disease. The patient faces the prospect of joining a clinical trial in Turkey, navigating not just health concerns but also the complexities of international travel and logistics.
This example underscores the critical role CROss play in streamlining patient recruitment and enrollment processes, particularly in trials with global reach. By leveraging their extensive network of investigators and trial sites, CROs facilitate quicker patient enrollment, which can significantly shorten study timelines, thus expediting the availability of potentially lifesaving treatments. Moreover, CROs bring to the table their in-depth knowledge of regulatory landscapes, which is essential in ensuring adherence to both local and international compliance standards, reducing the risk of costly non-compliance issues.
Their deployment of cutting-edge technologies and data management systems is vital for the integrity and precision of data collection and analysis. These advantages free up clinical trial companies to concentrate on their primary objectives and strategic initiatives, knowing that the CROss are adeptly managing the operational details. The collaborative efforts between clinical trial companies and CROs not only bolster efficiency but also extend the reach and impact of clinical research, ultimately benefiting patients who are in urgent need of innovative medical solutions.
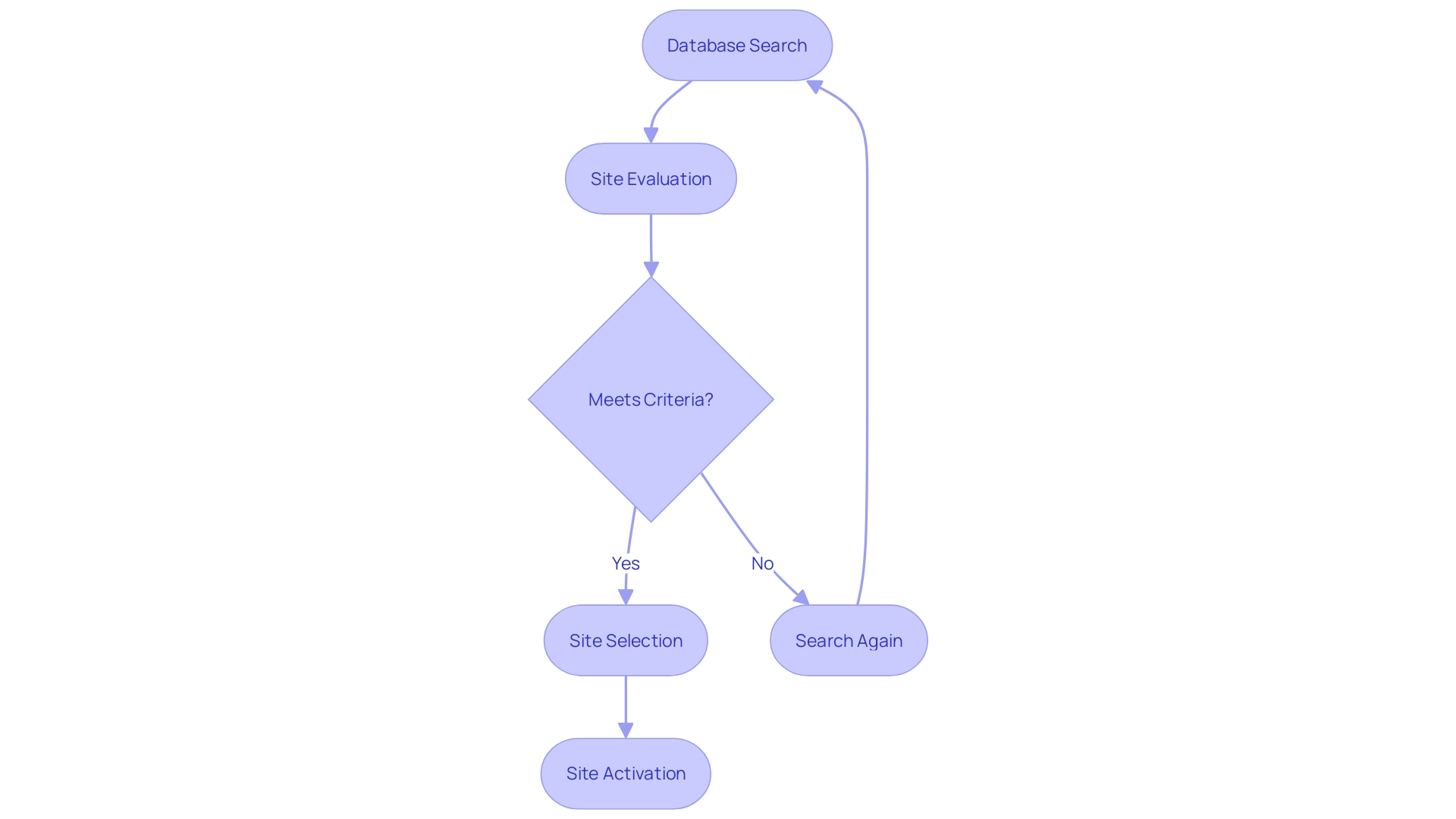
Strategic Site Identification and Activation
Clinical Research Organizations (CROs) are revolutionizing the selection and activation of trial sites through their adept use of advanced databases and established connections within the medical community. This expertise becomes particularly critical when patients with rare diseases, such as someone from rural Pennsylvania suffering from an illness without an FDA-approved treatment, are presented with the opportunity to join a trial abroad, for instance, in Turkey. The complexities of international travel, language barriers, and the navigation of foreign medical systems can be daunting for patients and their families.
Meanwhile, the advent of artificial intelligence (AI) and machine learning (ML) is propelling the healthcare industry forward, streamlining the orchestration of clinical trials with digital workflows and data management. The integration of these technologies is paving the way for highly personalized treatments and the concept of self-driving clinical trials, which could significantly enhance pharmacological research and patient care. With the burgeoning volume of health data, CROss are at the forefront of exploring these innovative approaches to ensure accuracy and efficiency in clinical trials, ultimately improving patient outcomes.
Streamlined Expertise and Cost Efficiency
Clinical trial companies often leverage the specialized expertise of Contract Research Organizations (CROs) to optimize the management and execution of clinical trials. CROs like CMIC Group, which initiated the CRO business in Japan over three decades ago, offer comprehensive services that span the full spectrum of the pharmaceutical value chain. With their end-to-end solutions, these organizations cater to the nuanced needs of pharmaceutical companies, medical device manufacturers, and research institutions.
The collaboration with CROss offers numerous benefits, including access to a breadth of professional experience in study design, monitoring, data management, and regulatory compliance, ensuring adherence to best practices and regulatory standards. Moreover, the cost-effectiveness of CRO partnerships is evident as they alleviate the need for dedicated in-house infrastructure and personnel, potentially leading to significant cost savings for clinical trial companies. This streamlined approach to clinical trial management not only bolsters the efficiency of the process but also facilitates the participation of patients, such as those from rural Pennsylvania facing rare diseases, in clinical trials globally, despite the logistical challenges of cross-border travel and language barriers.
Focus on Core Competencies and Risk Mitigation
Clinical trial companies enhance their operational efficacy by engaging with Contract Research Organizations (CROs), which specialize in managing the multifaceted aspects of clinical trials. This strategic partnership allows pharmaceutical entities to channel their expertise into drug development and scientific breakthroughs.
CROs like CMIC Group, with over three decades of pioneering experience in Japan, offer comprehensive services that span the entire pharmaceutical value chain. By leveraging such expertise, clinical trial companies can adeptly navigate the complexities of diverse trial environments and mitigate inherent risks.
For instance, consider a patient in rural Pennsylvania with a rare disease, who faces the opportunity to join a clinical trial in Turkey. The logistical challenges of international travel, language barriers, and document management are substantial. CROs are instrumental in facilitating these cross-border clinical engagements, ensuring that both the trial's integrity and the participant's needs are meticulously addressed, thereby streamlining the entire process for all stakeholders involved.
Global Reach and Regulatory Compliance
Clinical trial companies often face the complex challenge of conducting multi-national studies, which requires a broad understanding of various regulatory environments and the ability to manage logistical hurdles across borders. Contract Research Organizations (CROs) like CMIC Group, Japan's pioneering and largest CRO, offer comprehensive solutions that address these challenges.
With over three decades of innovation in the industry, CMIC provides an extensive array of services that cover the full spectrum of the pharmaceutical value chain, including essential support for multi-center trials. Their global reach, established through a network of partnerships and extensive knowledge of both local and international regulatory frameworks, is crucial for seamless trial execution.
For instance, a patient from rural Pennsylvania with a rare disease may face numerous obstacles when considering participation in a trial overseas, such as visa procurement, handling foreign documents, and coordinating travel. CROs are equipped to tackle these issues, thereby facilitating patient access to critical trials and ensuring regulatory compliance. Such end-to-end support not only mitigates the risk of delays but also enhances the prospects for successful trial outcomes across diverse regions.
Conclusion
In conclusion, Contract Research Organizations (CROs) are vital for the efficient management of clinical trials. They offer end-to-end solutions and specialized services that enhance the scope and effectiveness of clinical trial companies.
Outsourcing to CROs provides benefits such as streamlined patient recruitment and enrollment processes, quicker enrollment, and adherence to regulatory compliance standards. CROs excel in strategic site identification and activation through advanced databases and connections within the medical community.
The integration of artificial intelligence (AI) and machine learning (ML) further improves the orchestration of clinical trials. By partnering with CROs, clinical trial companies can focus on their core competencies in drug development while mitigating risks in diverse trial environments.
This collaboration enables efficient navigation of logistical challenges in cross-border clinical engagements. CROs also offer global reach through their extensive network of partnerships, ensuring smooth execution of multi-national studies. They facilitate patient access to critical trials while ensuring regulatory compliance. To summarize, CROs provide streamlined expertise, cost efficiency, global reach, and regulatory compliance for clinical trial companies. Their comprehensive solutions optimize operations and advance patient care by overcoming the challenges associated with clinical trial management.




