Introduction
Electronic Data Capture (EDC) systems are revolutionizing the landscape of clinical trials by providing an efficient, accurate, and regulatory-compliant method of data collection. These advanced platforms are critical in managing the voluminous and complex data generated during trials, which can include patient-reported outcomes, laboratory results, and imaging data. Utilizing EDC systems enables researchers to eliminate common data quality issues associated with paper-based collection, such as missing or ambiguous entries, and ensures data integrity by facilitating real-time data entry as mandated by trial protocols.
Moreover, the integration of EDC systems with digital health information systems exemplifies their pivotal role in enhancing healthcare delivery and managing public health concerns. The strategic application of EDC systems, underpinned by a robust data strategy, allows for the seamless assimilation of vast data sets from various digital sources, thereby empowering sponsors and clinical research organizations to derive actionable insights and make informed decisions in drug development. The adoption of EDC systems is indicative of a broader movement towards patient-centered research, where advanced methodologies and artificial intelligence are employed to harness the full potential of clinical data for the betterment of patient outcomes and the advancement of medical science.
What is an Electronic Data Capture (EDC) System for Clinical Trials?
Electronic Data Capture (EDC) systems are revolutionizing the landscape of clinical trials by providing an efficient, accurate, and regulatory-compliant method of data collection. These advanced platforms are critical in managing the voluminous and complex data generated during trials, which can include patient-reported outcomes, laboratory results, and imaging data. Utilizing EDC systems enables researchers to eliminate common data quality issues associated with paper-based collection, such as missing or ambiguous entries, and ensures data integrity by facilitating real-time data entry as mandated by trial protocols. Moreover, the integration of EDC systems with digital health information systems, as seen in Rwanda's national digitization strategy, exemplifies their pivotal role in enhancing healthcare delivery and managing public health concerns. This approach, which includes comprehensive electronic medical records, demonstrates a commitment to leveraging technology for superior patient care and streamlined clinical processes. The strategic application of EDC systems, underpinned by a robust data strategy, allows for the seamless assimilation of vast data sets from various digital sources, thereby empowering sponsors and clinical research organizations to derive actionable insights and make informed decisions in drug development. The adoption of EDC systems is indicative of a broader movement towards patient-centered research, where advanced methodologies and artificial intelligence are employed to harness the full potential of clinical data for the betterment of patient outcomes and the advancement of medical science.
Advantages of Using EDC Systems in Clinical Trials
Electronic Data Capture (EDC) systems have revolutionized the landscape of clinical trials by offering a suite of advantages that far surpass traditional paper-based methods. The use of EDC systems facilitates a more efficient study startup process, which is critical given the findings from a 2023 Study Activation Survey Report that highlights site set-up and training on sponsor-provided technology systems as the most burdensome activity during trial initiation.
Transitioning to EDC systems effectively addresses these burdens by streamlining data collection, reducing errors, and enhancing transparency, which is especially beneficial as clinical research sites report that the challenges with sponsor-provided solutions have increased over the past five years. With only 8% of clinical trials reaching successful completion, the shift towards EDC systems is a pivotal move to improve the efficiency and success rate of clinical trials.
Moreover, EDC systems support patient-centered drug development by enabling the integration of data from connected devices, wearables, and electronic diaries. This integration allows for the capture of comprehensive data insights, including patient behavioral trends that inform smarter drug development decisions. However, the vast amounts of data generated necessitate a well-defined data strategy to ensure the integrity and quality of data, which in turn supports patient safety.
Concerns around data quality are mitigated by EDC systems, as they eliminate common issues associated with paper-based data collection such as missing, ambiguous, or conflicting data. This is exemplified by a study where 44% of patients using the SF-36 quality-of-life questionnaire encountered issues in data entry. EDC systems also ensure that data is captured within the appropriate recall period as required by the protocol, preserving data integrity.
The adoption of EDC systems aligns with the growing emphasis on incorporating real-world data (RWD) into clinical trials, as highlighted by recent FDA guidance. The integration of RWD with clinical trial data through EDC systems fosters a more representative and accurate understanding of how drugs will perform in real-world settings.
In conclusion, EDC systems are a cornerstone in the modernization of clinical trials. They play a crucial role in alleviating the technological burden on sites, promoting data integrity, and supporting the seamless integration of RWD, ultimately leading to more successful clinical outcomes and advancements in patient care.
Streamlined Data Collection Process
Electronic Data Capture (EDC) systems are revolutionizing clinical trials by offering robust electronic case report forms (eCRFs) that enhance the user experience through intuitive design and functionality. These platforms facilitate direct data entry by researchers and healthcare professionals from a diverse array of organizations, such as hospitals, health systems, ambulatory care providers, and laboratories. Not only does this approach promote real-time validation and immediate resolution of discrepancies, but it also addresses data quality issues prevalent with traditional paper-based methods. Studies, including those at renowned institutions like Memorial Sloan Kettering Hospital, have underscored the significance of data accuracy and integrity which EDC systems help maintain by minimizing human error during data transfer processes. The economic advantage is also clear: adopting these technologies can lead to substantial cost savings in research and development by streamlining data management and reducing the manual effort required.
In the context of data strategy, it is imperative for sponsors and clinical research organizations to outline a comprehensive plan prior to protocol design. This involves selecting the optimal mix of traditional and digital data sources to inform decisions while prioritizing patient safety and data quality. The strategy should account for the various platforms and applications needed to access, manipulate, and analyze data, ensuring that the chosen EDC system aligns with the overarching goals of the project. By leveraging the power of artificial intelligence and connected devices, researchers can distill vast amounts of data from multiple sources into actionable insights, ultimately fostering patient-centered drug development.
Furthermore, the integrity of patient-reported outcome measures (PROMs) is enhanced when captured through EDC systems, which can mitigate the common pitfalls of paper diaries, such as missing, ambiguous, or superfluous data. Implementing an EDC system that supports the data review expectations across management, safety, clinical, and biostatistics teams can streamline the entire process, allowing for effective risk oversight and alleviating the burden on clinical sites.
Ultimately, the integration of EDC systems into clinical trials represents a paradigm shift in how data is collected, managed, and utilized, promising improved outcomes for patients, providers, and payers alike.
Improved Data Quality
The implementation of Electronic Data Capture (EDC) systems in clinical research has revolutionized the way data is collected, validated, and analyzed. EDC systems help mitigate common data collection issues such as transcription errors and incomplete data entries. With advanced validation protocols, these systems ensure that each data point is within pre-defined acceptable ranges, thus enhancing the accuracy and reliability of the data pool.
For example, in the context of patient-reported outcomes measures (Proms), where patients might collect data in unsupervised settings like their homes, EDC systems address data quality concerns. Studies have shown that traditional paper-based methods can result in significant data integrity issues, such as a study using the SF-36 quality-of-life questionnaire, which reported that 44% of patients either missed or ambiguously marked an item. EDC systems eliminate such issues by prompting for all necessary fields and validating the input in real-time.
Furthermore, recent advancements in data strategy have highlighted the importance of integrating traditional and digital data sources, ensuring comprehensive data collection that aligns with protocol designs. The value of data insights, especially when extracted from large volumes of clinical test data through artificial intelligence-driven methodologies, cannot be overstated. These insights offer a deeper understanding of patient behaviors and trends, which is crucial for informed decision-making in drug development.
The industry's move towards patient-centered drug development underscores the significance of capturing data from an array of sources, including connected devices and wearables. The EDC systems' ability to assimilate and validate data from these varied streams is a testament to their robustness and versatility in clinical research. In the PROTEUS study, for example, which involved over 2,300 patients across UK sites, EDC software was used in a randomized controlled trial for the first time for stress echocardiography, demonstrating its utility in diverse research settings.
Incorporating EDC systems is not only about enhancing data quality but also about creating an infrastructure that supports the integrity and traceability of data from collection to database storage. As emphasized by industry experts, understanding the origin of data and ensuring its recency are critical components of reliable research outcomes. EDC systems offer this traceability, allowing researchers to maintain a record of data transformations throughout the research process.
In light of these considerations, the integration of EDC systems is invaluable for clinical researchers aiming to achieve the highest data quality standards. By leveraging such innovative solutions, researchers can focus on the core aspects of their studies, knowing that the data they collect is both accurate and compliant with the highest integrity standards.
Enhanced Data Security and Authenticity
Electronic Data Capture (EDC) systems are revolutionizing the way clinical trials and research are conducted by providing robust data security and ensuring the authenticity of clinical data. EDC systems offer secure storage solutions, safeguarding sensitive information against loss and unauthorized access, which is critical in the clinical research environment where the protection of patient data is paramount. The significance of incorporating such secure systems is underscored by recent cybersecurity incidents, such as the ransomware attacks on critical services across the U.S., highlighting the vulnerability of organizations to digital threats.
One of the key features of an EDC system is the ability to meticulously record and timestamp every data entry, creating a comprehensive audit trail. This not only enhances data integrity but also ensures compliance with stringent regulatory standards, which require detailed documentation of all clinical trial activities. The importance of compliance is further emphasized by the guidance provided in 62443-2-5, which specifies the requirements for an effective cyber-security management system, tailored for end users and asset owners.
Ensuring data integrity is not just a regulatory necessity; it's a business imperative. The damage from data breaches extends beyond financial loss to include erosion of customer trust. A case in point is the East Texas hospital network incident, which disrupted critical emergency services due to a cybersecurity breach. EDC systems are designed to address these security challenges by integrating advanced features that protect against such vulnerabilities.
In today's data-driven world, where cyberattacks pose a significant threat to all sectors, the adoption of EDC systems is a strategic move to safeguard against the ever-evolving landscape of cyber threats. As stated by cybersecurity experts, 'All data isn't created equal, some data is special, and needs to be treated that way.' Thus, in clinical research, where data is not only sensitive but can have life-altering implications, the adoption of EDC systems represents a commitment to maintaining the highest standards of data security and patient privacy.
Key Features of EDC Systems
Electronic Data Capture (EDC) systems are integral to modernizing the clinical trial process, which has traditionally been hindered by slow, paper-based methods. EDC systems offer a suite of features designed to streamline study startup, enhance data integrity, and improve patient engagement. A pivotal feature is electronic consent (eConsent), which revolutionizes the way trial participants receive and understand information. Through multimedia elements, interactive glossaries, and knowledge checks, eConsent ensures patients fully comprehend the trial's scope, risks, and their role, significantly enhancing the informed consent process.
Furthermore, EDC systems address data quality concerns associated with paper-based patient-reported outcome measures (PROMs). When PROMs are collected electronically, issues with missing, ambiguous, or superfluous data are minimized, helping maintain the integrity and reliability of trial results. Additionally, the incorporation of digital health technologies (DHTs) in decentralized clinical trials (DCTs) allows for the collection of health care information directly from participants. Portable devices such as activity trackers, glucose monitors, and blood pressure monitors, facilitate remote data capture, contributing to a more patient-centric approach.
The adoption of EDC systems is not only a response to the cumbersome technology set-ups reported by clinical research sites but also an effort to embrace the shift towards DCTs. With the global decentralized clinical trial market projected to grow substantially, the use of EDC systems is critical in addressing the challenges of standardization, data security, and technological accessibility. As we move forward, the integration of EDC systems in clinical trials promises to enhance efficiency, reduce the technology burden, and pave the way for more successful outcomes in drug development.
Data Collection Methods and Integration
Electronic Data Capture (EDC) systems are revolutionizing the landscape of clinical trials and research, serving as a backbone for data aggregation and management. These systems enable a range of data collection methods, which include not only direct data entry but also cutting-edge approaches like electronic patient-reported outcomes (ePRO) and electronic clinical outcome assessments (eCOA). The integration capabilities of EDC systems are particularly noteworthy. They can seamlessly connect with a plethora of other vital systems, such as electronic health records (EHRs), laboratory information management systems (LIMS), and randomization and trial supply management (RTSM) systems. This interoperability ensures a smooth and uninterrupted flow of data across different platforms, enhancing the efficiency and effectiveness of clinical research operations.
Contributions to these systems come from a diverse spectrum of organizations which include hospitals, health systems, ambulatory care providers, and even pharmacies. This diversity ensures that a wealth of data, ranging from patient health records to laboratory test results, is captured and organized in a manner that upholds the integrity of research findings. The strength of EDC systems lies in their ability to mitigate data quality issues common with paper-based methods, such as missing or ambiguous entries. For example, studies have highlighted that paper-based collection of quality-of-life questionnaires like the SF-36 can result in data ambiguities in as much as 44% of cases. This is a stark contrast to EDC systems, which not only ensure data clarity but also adherence to protocol-specified timing for data entry, thereby maintaining data integrity.
The financial outlay associated with implementing and utilizing EDC systems is an investment in quality and security. While there are costs involved, the return on investment comes in the form of high-quality data that meets regulatory standards and the potential for significant advancements in medical research. Moreover, these systems are designed to be compatible with various data platforms and applications, making them accessible and user-friendly for researchers.
As clinical trials evolve with the introduction of decentralized clinical trials (DCTs) and digital health technologies (DHTs), EDC systems stand at the forefront of this transformation. They are integral in capturing health care information directly from individuals using a range of devices from activity trackers to blood pressure monitors. The global market for DCTs is on a substantial growth trajectory, expected to expand at a compound annual growth rate of 30.1% from 2021 to 2026, indicating the critical role of EDC systems in the future of clinical research.
In summary, EDC systems offer a robust framework for data management in clinical trials, addressing the challenges of data quality and integrity while fostering an environment for high-impact research. Their role in streamlining data collection processes, ensuring compliance, and supporting the expansion of DCTs is undeniable, marking them as indispensable tools in the pursuit of medical advancements.
Configurability and Customization
Electronic Data Capture (EDC) systems have become a cornerstone in modernizing the clinical research landscape, offering unparalleled flexibility and adaptability. These systems are engineered with the capability to be tailored to the distinct requirements of diverse clinical trials. Research teams have the freedom to create and adjust electronic Case Report Forms (eCRFs), refine data validation protocols, and devise data entry interfaces that precisely gather essential data elements. This customization is critical given the diverse nature of clinical trial sites, such as academic medical centers, non-academic centers, and professional site networks, each with their own unique operational challenges and technological proficiencies.
In light of the exigencies presented by global health crises like the COVID-19 pandemic, the clinical trial process has come under scrutiny for its slow pace, compounded by a mere 8% success rate of trials. With the industry calling for patient-centered drug development, there's a push to harness the power of connected devices and advanced AI-driven analytical methods. This approach enables the extraction of valuable insights from a myriad of data sources, including lab results, patient-reported outcomes, and imaging. Decentralized clinical trials (DCTs), leveraging digital health technologies such as wearables and remote monitoring devices, are at the forefront of this innovation. However, the integration of such technologies introduces complexities in data management that necessitate a well-defined data strategy prior to the protocol design stage.
The 2023 Study Activation Survey underscores the importance of alleviating the technological burdens on clinical research sites. With sites reporting that setup and training on sponsor-provided systems are more taxing than contracting and budgeting, it is imperative that the industry moves towards offering a more flexible technology ecosystem. This ecosystem should accommodate sites with varying levels of technological expertise while ensuring seamless integration and flow of data and workflows. As we strive for efficiency and transparency in clinical trials, the role of EDC systems is more significant than ever, streamlining processes and reducing friction in study startups, ultimately accelerating the path to innovative medical solutions.
Real-Time Data Monitoring and Patient Safety
Electronic Data Capture (EDC) systems have revolutionized the landscape of clinical research by introducing an unprecedented level of efficiency and accuracy in data management. Providing real-time data monitoring, these systems have become indispensable in the modern healthcare setting. They offer a suite of tools that enable researchers and healthcare professionals to maintain the highest standards of patient safety and data integrity. One key feature is the generation of instant alerts and notifications that flag data inconsistencies or adverse events, thus facilitating swift intervention.
For example, ADRZ, a regional hospital in The Netherlands, relies on a robust operating network to support its 24/7 healthcare services. The scalability of their systems ensures that a growing number of users and devices can be accommodated without compromising performance. This is crucial in a setting where real-time data can make a difference in patient care and outcomes. Similarly, CHS, a large provider organization in the United States, sought solutions that were evidence-based and scalable to reduce patient falls during challenging times marked by staffing shortages and nurse burnout.
EDC systems also play a critical role in enhancing patient safety measures such as fall prevention and emergency nurse response. By integrating real-time analytics, healthcare institutions can process and analyze data immediately as events occur. This capability is exemplified by the use of Real-Time Location Systems (RTLS) to prevent patient wandering, a common risk in patients with dementia or those disoriented due to illness.
Furthermore, in the context of quality improvement, EDC systems help in harnessing the power of real-time patient electronic health record (EHR) data. For instance, the Epic Deterioration Index (EDI) is a machine learning model that predicts clinical deterioration. Despite mixed results in studies on the effectiveness of these early warning scores (EWSs) in patient outcomes, their rapid adoption underscores the healthcare industry's commitment to leveraging technology for better patient care.
In addition to these operational benefits, EDC systems contribute to the advancement of clinical research by providing reliable and timely data. A recent collaboration highlighted this, where Clario's wearable sensor technology was used in a University of Oxford study to monitor Parkinson's disease progression. With the remote patient monitoring market projected to grow significantly, the importance of EDC systems in facilitating these advances cannot be overstated.
In summary, EDC systems are not merely tools for data collection; they are central to a proactive approach in patient care and safety, enabling healthcare providers to deliver timely interventions and informed decisions, thereby optimizing patient outcomes and advancing clinical research.
Data Blinding and Ethical Considerations
EDC (Electronic Data Capture) systems play a pivotal role in safeguarding the integrity and ethical standards of clinical trials. By implementing data binding, EDC systems ensure the concealment of treatment assignments from both researchers and participants, thereby minimizing potential bias and preserving the study's validity. Furthermore, these systems are designed to protect patient privacy through data de-identification and anonymization techniques. These methods break the direct link between personal data and the individuals they pertain to, mitigating privacy risks associated with the handling of sensitive information such as medical records, biometric, or genetic data.
Adherence to ethical guidelines is not merely about compliance with regulations; it reflects a commitment to principles such as honesty, accountability, and professional fairness. An inclusive approach, as suggested by the Singapore Statement, calls for proactive strategies that uphold research integrity beyond the mere avoidance of misconduct, which encompasses fabrication, falsification, and plagiarism. EDC systems, therefore, are not just tools for data collection but are instrumental in fostering an environment where research is conducted with the highest ethical standards.
Selecting the Right EDC System for Your Clinical Trial
The landscape of clinical research is rapidly evolving, with an increased emphasis on patient-centered drug development. This evolution is powered by a surge in data insights accessible through connected devices, wearables, electronic diaries, and other decentralized trial solutions. The integration of advanced artificial intelligence methodologies is further enhancing our ability to glean significant insights from diverse data types such as lab results, patient reports, and imaging. The potential data within a single study can now expand at an exponential rate.
In the face of such data abundance, the selection of an appropriate Electronic Data Capture (EDC) system becomes pivotal. The chosen EDC must not only handle the volume but also support the complexity of data, ensuring it can be collected, monitored, cleaned, and analyzed efficiently. The right EDC system should facilitate a cohesive data strategy that is determined before protocol design, aiming to optimally gather information from both traditional and digital sources as the trial progresses. This strategic approach to data management is essential to harness insights while maintaining patient safety and data quality.
Key considerations for managing the data flow in an EDC system involve addressing several critical questions. What are the expectations for data review across various domains such as data management, safety, and biostatistics? Does the data strategy meet these expectations? How will the data flow strategy support study risks mitigation and enable risk oversight through data insights? Moreover, the impact of the data flow strategy on sites, including potential burdens, must be evaluated to ensure smooth operation.
Real-world case studies, like the Orchestras study managed by Curavit for MedRhythms, underscore the importance of selecting the right EDC system. The study focuses on evidence generation and the financial impact of novel healthcare technologies, necessitating a tech-enabled approach for efficient trial management. Similarly, the MARRS study represents the application of an EDC system in a multi-center setting, highlighting the critical role of EDC systems in large-scale clinical trials.
As reported by the Editor in Chief of the Journal of Clinical and Translational Science, the global decentralized clinical trial market is forecasted to grow significantly, necessitating robust EDC systems to support these innovative trial designs. With a projected compound annual growth rate of 30.1% from 2021 to 2026, EDC systems must evolve to meet the demands of decentralized clinical trials, including the handling of complex data flows and ensuring data security and privacy.
Ultimately, selecting the right EDC system is not a trivial task. It demands a thorough understanding of the trial’s data strategy, the complexities of data flow management, and the need to meet regulatory and ethical standards. By carefully considering these factors, clinical researchers can equip themselves with powerful tools to manage the wealth of digital data, driving smarter decisions in drug development.
Considerations for EDC System Selection
Selecting an EDC system necessitates careful consideration of various factors to ensure data integrity, security, and compliance with regulatory standards. Digital security is paramount; a robust EDC system should incorporate advanced security measures such as multi-factor authentication and data encryption to protect against unauthorized access and cyber threats. Furthermore, the system's mobile application should allow for seamless management of trial data, with timely updates and alerts to maintain the integrity of the research.
Cost is another critical consideration. The investment in an EDC system includes not only the initial outlay for equipment but also the ongoing expenses for system maintenance and updates. It's important to evaluate the total cost of ownership when comparing different EDC systems.
Customer reviews and real-world experiences offer valuable insights into the performance and reliability of an EDC system. Prospective users should look for reviews from verified users, which often include detailed accounts of system usability, customer support responsiveness, and the overall impact on the efficiency of research activities.
Supporting these considerations, recent news highlights the importance of innovative technology in clinical trials. For instance, a clinical evaluation set to include 40 adult participants underscores the potential of technology to revolutionize patient monitoring in clinical settings. Another study, Orchestras, emphasizes the necessity of managing complex digital clinical trials effectively, showcasing the importance of an EDC system in facilitating such efforts.
In conclusion, when selecting an EDC system, it's essential to consider digital security, costs, and customer reviews, along with insights from recent industry advancements. These factors will guide researchers to a solution that meets the rigorous demands of modern clinical trials, ensuring the collection of high-quality data while safeguarding participant privacy and study integrity.
Role of Contract Research Organizations (CROs) in EDC Selection
Contract Research Organizations (CROs) are indispensable allies in the clinical trial ecosystem, particularly when it comes to the deployment of Electronic Data Capture (EDC) systems. Their proficiency lies in meticulously evaluating EDC vendors and their offerings, ensuring that the systems align with the nuanced demands of various clinical trials. Drawing on a rich history, such as that of CMIC Group in Japan, which has over three decades of experience and a comprehensive understanding of the pharmaceutical value-chain, CROss bring a wealth of knowledge to the table. They don't just assess the technical capabilities of EDC solutions but also provide bespoke recommendations that resonate with the specific needs of a trial.
This deep expertise is crucial in a landscape where clinical research faces a workforce crisis, with a staggering demand for skilled professionals outpacing supply. CMIC's tailored approach exemplifies how CROs can alleviate some of these pressures by offering end-to-end services that span the entire drug development lifecycle. Furthermore, innovation in health technology, as seen in recent advancements like brain-computer interfaces and tongue-controlled devices, underscores the need for EDC systems that can adapt to new types of data and complex trial designs.
The importance of selecting the right EDC system is underscored by recent studies, such as the OrcHESTRAS trial, which highlight the economic and patient care implications of clinical research. With the decentralized clinical trial market projected to grow significantly, the role of CROs in guiding EDC selection becomes even more pertinent, ensuring trials are not only patient-centric but also fiscally responsible. By leveraging their expertise, CROss are pivotal in facilitating efficient, high-quality data collection and management, ultimately advancing the field of clinical research.
Implementation and Training
Selecting the right Electronic Data Capture (EDC) system for clinical trials is just the beginning; its successful implementation and user training are equally critical. Ensuring that the chosen EDC system is seamlessly integrated into the research workflow is paramount. This involves converting complex data tables and schemes into user-friendly formats that encourage engagement and self-guided exploration. For instance, Nets, a provider of digital payment solutions, faced a similar challenge. They revolutionized their onboarding process by transforming technical data into more digestible and interesting formats, thereby motivating users to self-learn.
When it comes to training, it's essential to provide comprehensive instruction on the EDC system's functionalities. This is akin to the approach in the financial sector, where companies are often required to train their staff on international regulations, such as fraud protection and Anti-Money Laundering. Effective training equips users with the best practices for data management and ensures that they can navigate the system confidently and efficiently.
Moreover, as we face an increasingly sophisticated array of digital threats, it's clear that an in-depth understanding and strategic approach are necessary. This involves not just knowing about cyber attacks but understanding the attackers' methods and motivations. Similarly, users of an EDC system must be educated to recognize and respond to data management challenges proactively. The goal is to safeguard critical research data and maintain the integrity of clinical trials, much like how robust Cyber Threat Intelligence (CTI) programs protect organizations.
Compliance and Security
At the cornerstone of effective clinical research is the pivotal role of Electronic Data Capture (EDC) systems. These systems must not only streamline data collection but also ensure rigorous adherence to regulatory standards, such as Good Clinical Practice (GCP) and data protection regulations. In the context of increasing technological integration, with wearables, IoT devices, and mobile technologies becoming commonplace, the importance of data security has been magnified. The recent FDA mandate exemplifies this, allowing the FDA to approve or reject medical devices based on cybersecurity considerations, thus reinforcing the critical nature of secure and compliant data management in EDC systems.
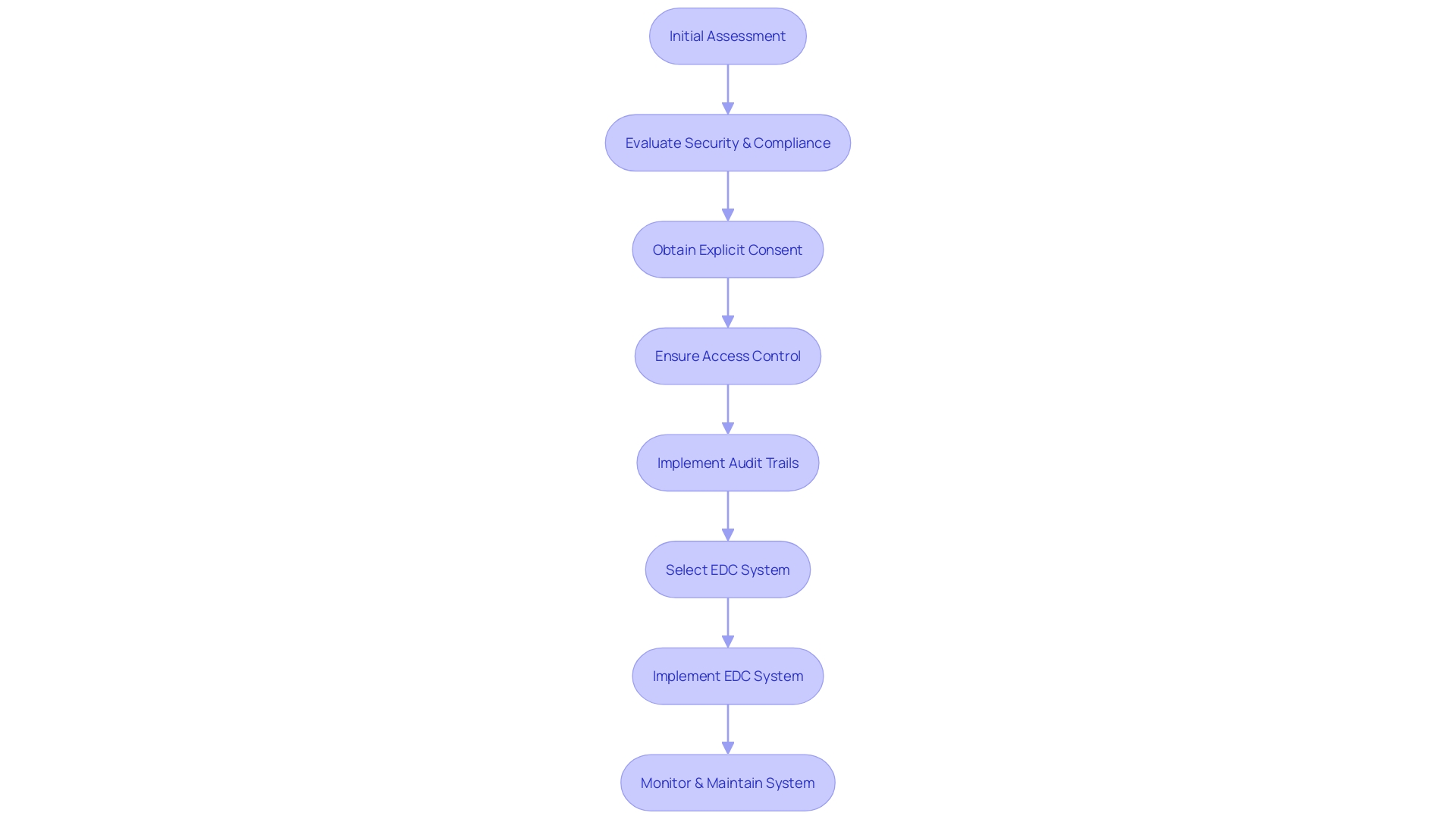
In light of these requirements, the process of selecting and implementing an EDC system involves meticulous assessment. For instance, the Digital Service Team at healthcare institutions conducts thorough evaluations to ascertain the security and compliance of potential technologies. This includes an initial assessment to determine if the requested technology aligns with existing solutions and meets the stringent standards for data protection. As Carrie Gluck, CISO at Rectangle Health, emphasizes, establishing trust through secure and compliant systems is integral to healthcare services. The emphasis on obtaining explicit consent before data collection further solidifies the commitment to data privacy and the respect for patient autonomy.
Moreover, the dynamic regulatory landscape necessitates that organizations remain vigilant and proactive in their digital assurance processes. With the variability of systems used across the industry, from government to site-owned platforms, the challenges are multifaceted. Ensuring access control, functionality adequacy, and the provision of direct access and comprehensive audit trails are essential components of this ongoing effort. As articulated by industry experts, overcoming these challenges involves a series of steps from scoping and planning to testing and validation, highlighting the complexity of implementing systems that stand up to the evolving expectations of guidelines and regulations.
Cost Implications and ROI
When considering the integration of Electronic Data Capture (EDC) systems in clinical trials, it's crucial to acknowledge the initial investment and gauge the potential return on investment (ROI). EDC systems, while requiring upfront expenditure, offer a pathway to long-term efficiency gains and cost reductions by enhancing data precision, expediting analysis, and diminishing the incidence of errors. One must look no further than institutions like ADRZ in The Netherlands, which has benefitted from a stable network infrastructure supporting its expansive care provision, or Somerset Academies of Texas, which streamlined multiple software systems into a cohesive whole, to see the transformative impact of such technology integrations on operational efficiency.
Moreover, the evolution of digital therapeutics underscores the importance of leveraging digital platforms in healthcare. These therapeutics, while not extensively used due to their novelty and insurance coverage nuances, have demonstrated the potential to reduce patient costs significantly, indicating a similar promise for EDC systems in managing clinical trial expenses.
The burgeoning field of decentralized clinical trials (DCTs), which is projected to grow at a 30.1% compound annual growth rate from 2021 to 2026, further accentuates the need for robust EDC systems. DCTs introduce challenges such as ensuring data privacy, maintaining participant engagement without coercion, and overcoming technological literacy barriers. Developing best practices and standardized approaches to these trials is fundamental, as is the adoption of technologies that support quality results and extend trials to underserved regions.
Strategically, the aggregation of data from various sources, including connected devices and wearables, necessitates a well-defined data strategy prior to protocol design. The goal is to ensure that data insights are maximized for informed decision-making, emphasizing patient safety and data integrity. This calls for a collaborative effort between sponsors and clinical research organizations to map out optimal data collection from a mix of traditional and digital data sources, as well as to address the critical considerations for managing data flow.
In summary, incorporating EDC systems into clinical trials is not merely an operational decision but a strategic move towards enhancing data management, improving patient engagement through electronic consent mechanisms, and ensuring the integrity and quality of trial outcomes. The foresight to anticipate the complexities of data integration and the subsequent design of data strategies can mitigate risks, meet regulatory expectations, and ultimately provide a substantial return on investment.
Conclusion
In conclusion, Electronic Data Capture (EDC) systems have revolutionized clinical trials by providing an efficient, accurate, and regulatory-compliant method of data collection. These systems eliminate common data quality issues associated with paper-based collection, ensuring data integrity and real-time data entry as mandated by trial protocols. The integration of EDC systems with digital health information systems enhances healthcare delivery and public health management.
Advantages of using EDC systems in clinical trials include streamlining study startup, reducing errors, and enhancing transparency. They support patient-centered drug development by integrating data from connected devices and electronic diaries, providing comprehensive insights for informed decision-making. EDC systems also address data quality issues and align with the emphasis on incorporating real-world data into trials.
EDC systems offer streamlined data collection processes, enhancing the user experience and addressing data quality issues prevalent in traditional methods. Their integration represents a paradigm shift in data collection, management, and utilization, promising improved outcomes for patients, providers, and payers.
Implementing EDC systems enhances data accuracy, reliability, and traceability. They capture data from various sources, ensuring integrity and supporting comprehensive data collection.
EDC systems provide robust data security, safeguarding sensitive information and ensuring authenticity. They offer a comprehensive audit trail, enhancing data integrity and compliance with regulatory standards.
Key features of EDC systems, such as electronic consent and case report forms, streamline study processes, enhance data integrity, and improve patient engagement. They facilitate the integration of diverse data sources, supporting patient-centered drug development.
Selecting the right EDC system is crucial, considering factors such as data volume, complexity, and support for cohesive data strategies. EDC systems play a pivotal role in safeguarding the integrity and ethical standards of clinical trials.
Successful implementation and user training are essential for efficient utilization of EDC systems, ensuring seamless integration into the research workflow and comprehensive instruction on system functionalities.
EDC systems must ensure compliance with regulatory standards and data protection regulations, establishing trust through secure and compliant systems and upholding patient privacy.
In conclusion, EDC systems revolutionize clinical trials by enhancing data management, patient engagement, and trial outcomes. They streamline data collection, ensure compliance, and support the integration of diverse data sources, driving advancements in medical research and improving patient care.




