Introduction
Post-Market Clinical Follow-up (PMCF) is a critical aspect of medical device regulation under the Medical Device Regulation (MDR). It involves the continuous gathering, monitoring, and assessment of clinical data post-market release to confirm the safety and efficacy of medical devices. PMCF activities serve as a foundation for hypothesis generation in clinical studies and contribute to the retraining of AI/ML-enabled medical devices.
Real-world data gathered from PMCF is indispensable, as it helps prevent potential device-related complications and supports regulatory compliance. PMCF also addresses environmental responsibilities and ensures the proper disposal and recycling of electronic equipment. Ultimately, PMCF enhances patient outcomes and public health safety.
In this article, we will explore the key objectives, components, design, structure, implementation, evaluation, and reporting of PMCF. We will also discuss the challenges and best practices in implementing PMCF activities.
What is PMCF and Its Importance
Post-Market Clinical Follow-up (PMCF) is a crucial aspect of regulation under the Medical Device Regulation (MDR), encompassing an ongoing process that involves the collection, monitoring, and evaluation of clinical data related to a product post-market release. This ongoing evidence collection serves a dual purpose: confirming the safety and efficacy of the product and ensuring its continued performance across diverse real-life clinical situations.
The requirement for PMCF arises from the recognition that medical tools, once in the market, may face different challenges and usage scenarios not fully captured during pre-market trials. For example, modifications in equipment design due to customer feedback, adjustments in manufacturing materials or processes, or other factors require careful documentation, potentially resulting in additional regulatory submissions. These modifications are examined in relation to form, fit, and function—essential characteristics that define the components of the apparatus.
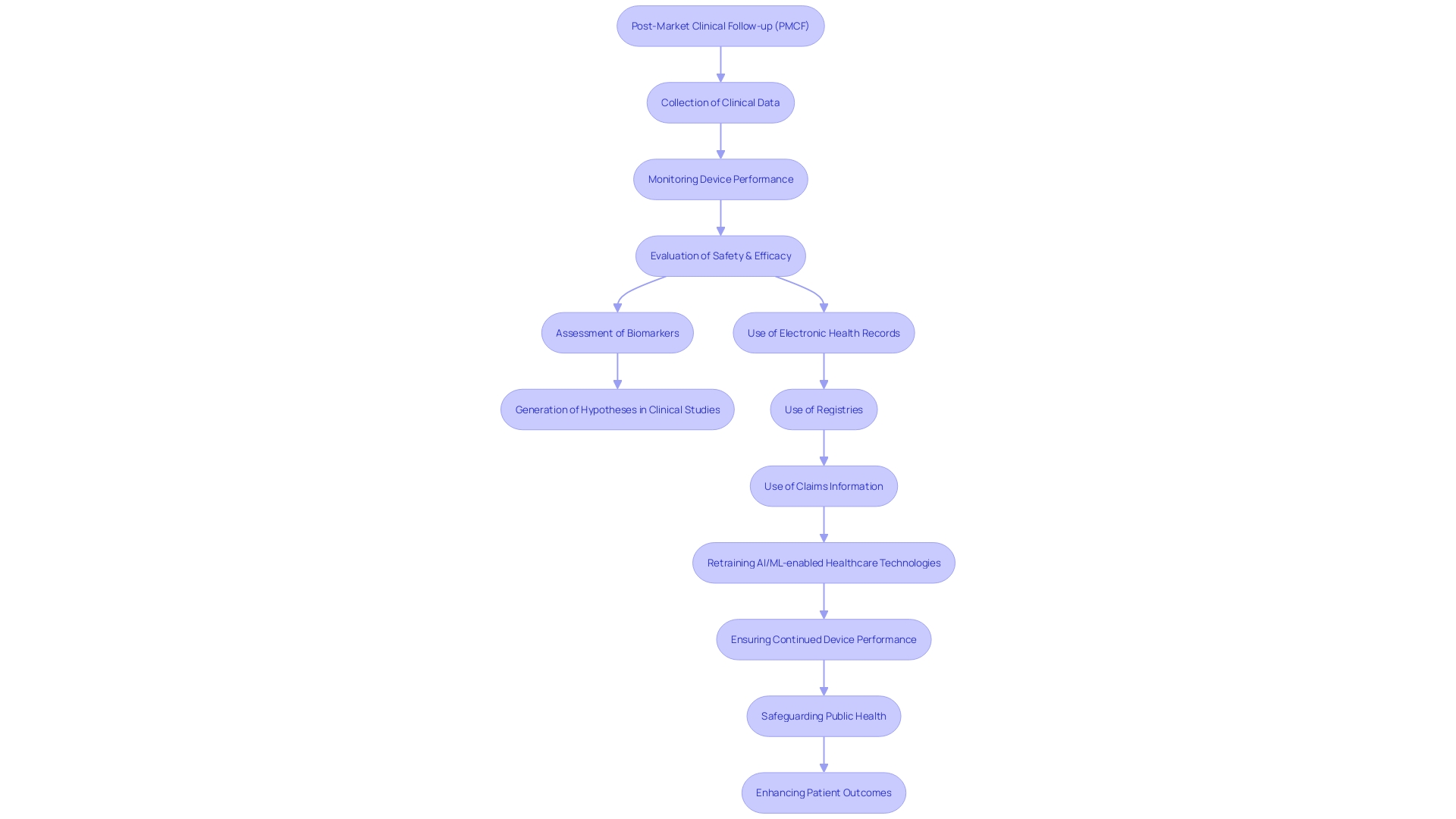
Furthermore, activities related to the post-market clinical follow-up, such as the assessment of a biomarker or clinical outcome measurement, provide a basis for generating hypotheses in clinical studies, offering a control group from previous studies or contributing information in a hierarchical model. In the field of post-market clinical follow-up, harnessing information from electronic health records, registries, or claims information is essential for retraining AI/ML-enabled healthcare technologies, guaranteeing their adaptability and continued effectiveness.
Data collected from real-life studies is crucial, as shown by the concerning fact that in the United States alone, more than 1.7 million injuries and 83,000 deaths may have been linked to faulty healthcare equipment over a ten-year period. The FDA is actively working to develop a surveillance system to monitor such safety issues, emphasizing the importance of post-market clinical follow-up in safeguarding public health.
Additionally, the process of post-market clinical follow-up is crucial in dealing with the requirements of regulations such as the WEEE, which mandate the appropriate disposal and recycling of electronic equipment, including healthcare devices. This ensures that manufacturers fulfill their environmental responsibilities, contributing to the reduction of pollution and resource depletion.
In essence, the process not only facilitates the early detection and prevention of potential device-related complications but also supports regulatory compliance and ethical standards, ultimately enhancing patient outcomes and public health safety.
Key Objectives of PMCF
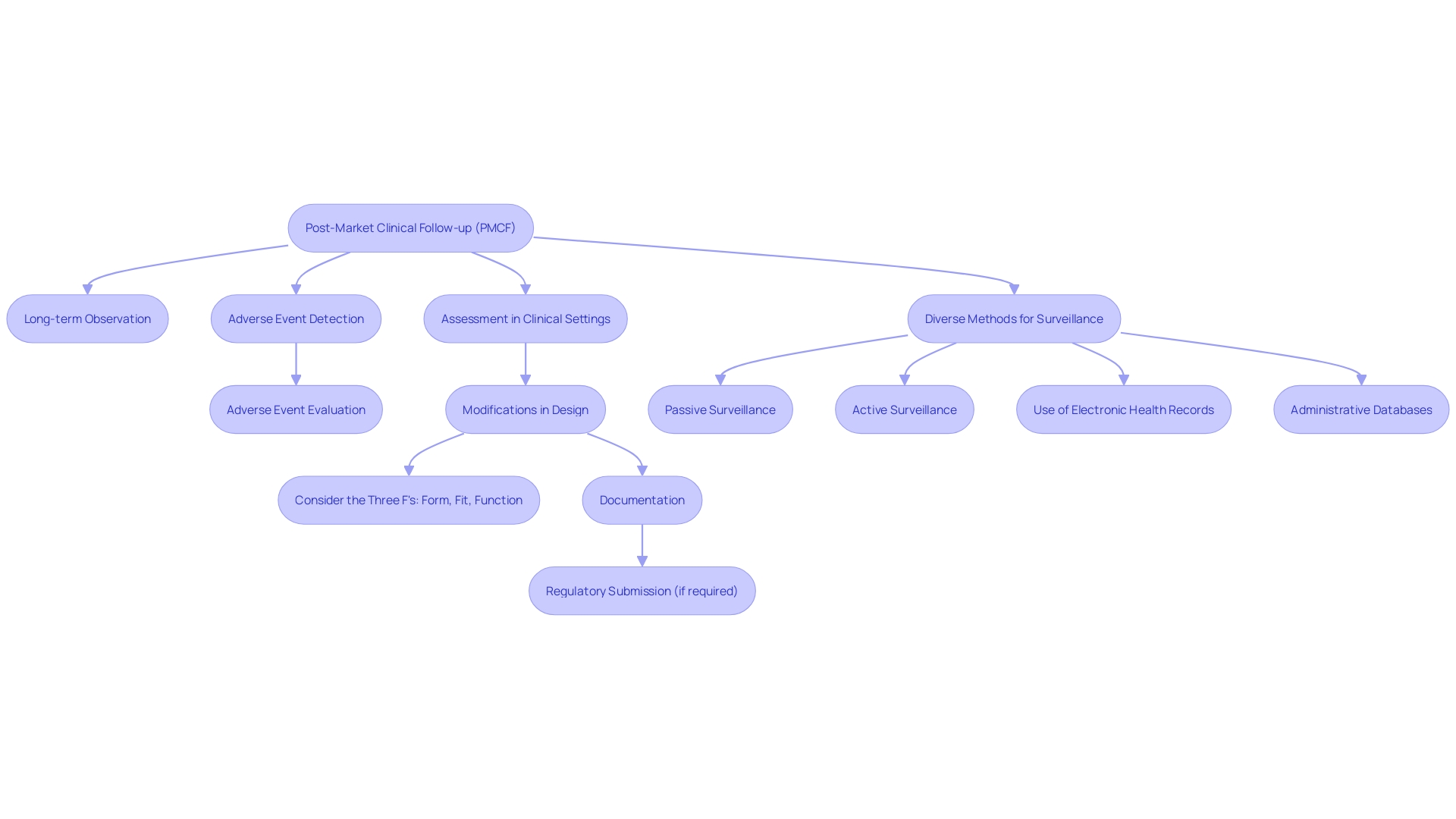
The vital objectives of Post-Market Clinical Follow-up (PMCF) involve a variety of activities essential for ensuring the safety and effectiveness of medical instruments once they are being utilized. These activities include careful long-term observation of equipment performance and safety, the diligent detection and evaluation of any adverse events or side effects, and the thorough assessment of equipment performance within real-world clinical settings. Post-Market Clinical Follow-up is crucial in discovering possible risks or issues that may not have been apparent during pre-market clinical trials. Moreover, it plays a crucial role in gathering further clinical evidence to support the safety and performance assertions of the equipment, thus facilitating ongoing improvement in our understanding of the equipment's advantages and hazards.
Real-world examples illustrate the dynamism of PMCF. For example, modifications made to the design of a product after it has been released to the market, in response to customer input or changes in materials and manufacturing procedures, must be carefully recorded and could require a fresh regulatory filing. This highlights the significance of comprehending the 'three Fs' - form, fit, and function - which delineate the critical attributes of a gadget's components.
In a time period where advancements in medical technology progress quickly, the process of Post-Market Clinical Follow-up (PMCF) serves as a safeguard, guaranteeing that alterations to medical instruments do not jeopardize the well-being of patients. A documentary titled 'The Bleeding Edge' highlighted that clinical trials are not always mandatory for devices seeking FDA approval through the 510(k) clearance process. This discovery enhances the importance of post-market surveillance, which functions as a continuous supervision mechanism in the post-market phase.
To effectively implement PMCF, diverse methods are employed, including passive and active surveillance systems. These involve spontaneous reporting by healthcare professionals and patients, as well as dedicated registries or studies, coupled with the analysis of electronic health records and administrative databases. Such vigilant monitoring is crucial, as evidenced by the association of more than 1.7 million injuries and 83,000 deaths with potentially faulty healthcare apparatus in the U.S. over a ten-year period.
The FDA stresses the significance of protecting public health by guaranteeing the safety and efficiency of healthcare equipment. This dedication is evident in the creation of a surveillance system to oversee potential safety concerns in healthcare instruments, an effort that is anticipated to grow gradually, despite obstacles in financing and patient recognition.
In summary, post-market clinical follow-up is not just a regulatory obligation; it is an essential element of medical device management that encourages creativity, improves safety, and supports international alignment in criteria and excellence, ultimately resulting in improved health results and the transformation of lives worldwide.
Components of a PMCF Plan
To guarantee the utmost quality of patient care and adherence to regulations, a strong plan for clinical follow-up after the product is on the market is crucial. This entails a strategic approach to information collection where specific data points and variables are meticulously defined for ongoing analysis and improvement. The plan for post-market clinical follow-up is not fixed; it must adjust to changing study designs that give priority to patient populations, sample size, and methodologies for collecting information that are responsive to technological advancements and patient requirements.
Examining the gathered data necessitates an all-encompassing statistical approach, which must be delineated in the plan for continuous product enhancement and patient well-being. Creating well-defined schedules and key points is essential, as it enables the organized monitoring of advancement and recognizes vital stages in the process of post-market clinical follow-up. This systematic approach not only aligns with the overarching objective of enhancing patient outcomes but also corresponds with the ISO 13485 standard's focus on quality management throughout the manufacturing process of healthcare equipment.
Moreover, allocating appropriate resources and budgeting is a fundamental component of the PMCF plan, ensuring that each phase is executed effectively. This resource allocation is crucial in advancing the product's journey from conception to market, all while maintaining a patient-first philosophy. It is a combination of conventional project management skills and the particular practices of the healthcare equipment industry that ultimately produces products that improve patient well-being and adhere to regulatory frameworks.
Recent regulatory updates, such as those from the UK's MHRA, demonstrate a global transition towards more patient-centered prerequisites for healthcare equipment. This developing regulatory scenario emphasizes the significance of a plan for post-market clinical follow-up that is not only in line with the regulations but also anticipates future standards and technological advancements. It is this progressive mindset that will sustain the momentum of innovation in healthcare instruments and guarantee that patient safety stays at the forefront of development and post-market activities.
Designing a PMCF System
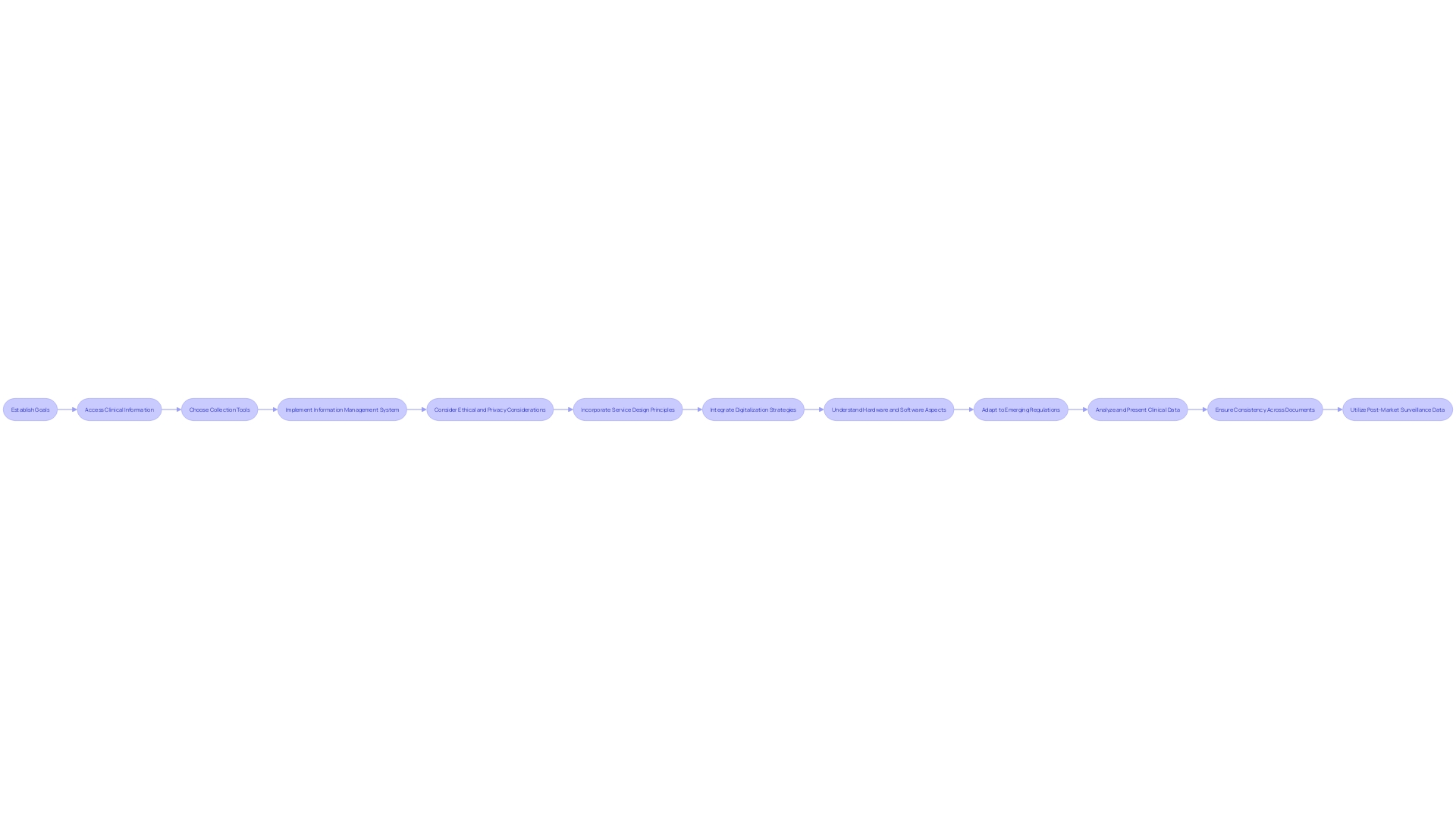
Developing a successful Post-Market Clinical Follow-up (PMCF) system is a complex procedure that combines state-of-the-art technology with rigorous regulatory requirements. It starts by establishing specific goals for the PMCF that are in line with the specific requirements of the medical equipment. Recognizing the origins of relevant clinical information is the subsequent vital phase, which may involve accessing registries, electronic health records, and existing post-market surveillance systems.
The choice of collection tools is also crucial and should be customized to the device and patient population, utilizing instruments such as surveys, patient questionnaires, or advanced remote monitoring technologies. A strong information management system is crucial to ensure the precision and reliability of the gathered information, as well as to safeguard patient confidentiality.
Ethical and privacy considerations must be strictly observed throughout the PMCF process. This adherence to ethical standards is not just a regulatory requirement but also a commitment to patient safety and the trustworthiness of the data collected.
Service design principles are crucial in this context, as they focus not only on patient needs but also on the wider ecosystem of healthcare providers who interact with the medical device. This comprehensive approach guarantees that all parties involved, such as doctors, nurses, maintenance personnel, and educators, are taken into account in the development of the system.
With the current emphasis on digital technology in healthcare, it is essential to integrate smart digitalization strategies. These strategies should focus on identifying key data and leveraging it to achieve not just desired outcomes but also those that are necessary for regulatory compliance and improved patient care.
The effectiveness of a system is based on a comprehensive knowledge of both the hardware and software aspects of medical devices, as well as the intricate interaction between them. This includes considerations of how images produced by the device interface with software development and the user experience of patients and healthcare professionals.
In today's changing regulatory environment, demonstrated by the recent extensive AI guidelines from the Biden administration and the ongoing debates about the AI Act in Europe, the significance of creating a system for post-market clinical follow-up that can adjust and adhere to emerging regulations cannot be emphasized enough. Furthermore, the utilization of digital certificates and the functions of Certificate Authorities (Cas) in establishing trust and authenticity in digital systems emphasize the necessity for secure and verifiable information within the framework.
By integrating these varied components - from service design to digitization, and from data control to regulatory anticipation - the formation of a system for post-market lifecycle management can greatly improve the maintenance of healthcare instruments, guaranteeing that they consistently adhere to the utmost levels of safety and effectiveness.
Structuring a PMCF Plan
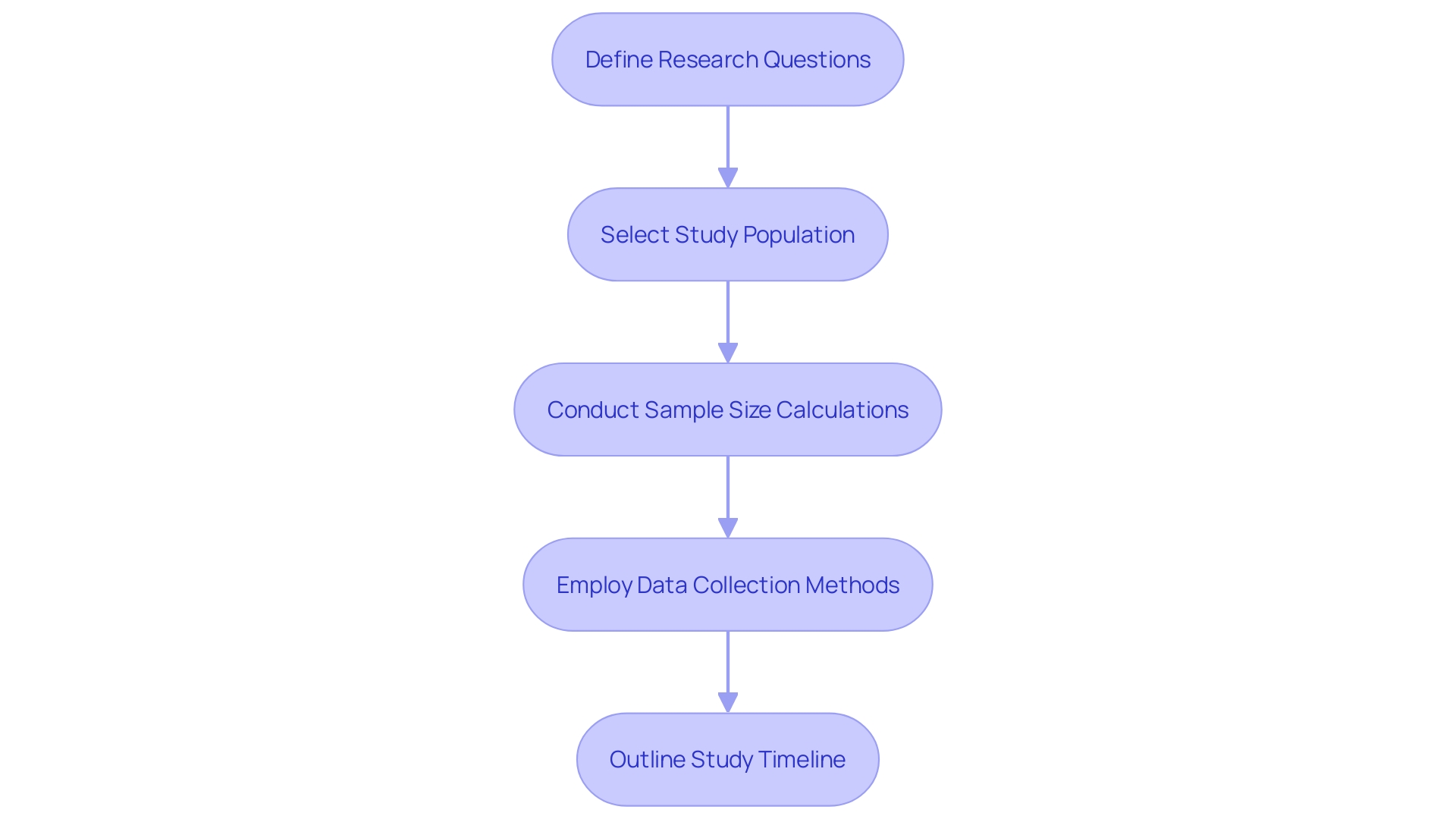
When developing a plan for the clinical follow-up after the product is released to the market, the procedure must be carefully organized to guarantee the ongoing assessment of the device's effectiveness and safety. Firstly, the plan should start by defining precise research questions that are tailored to the PMCF's objectives, which might include assessing long-term effectiveness or identifying unforeseen risks. Selection of the study population is critical; it must be representative of all end users, such as patients across diverse medical conditions, healthcare professionals, and administrative staff, to yield valid and generalizable results.
A robust sample size calculation is paramount to achieve statistical power and validity. This calculation should be informed by the expected differences in outcomes, the variability within the population, and the level of precision desired for the study results. For information gathering, a range of approaches should be employed that are not only suitable for the population but also capable of capturing the necessary information effectively. These methods could involve patient surveys, clinical assessments, and the use of healthcare databases.
Furthermore, a comprehensive study timeline is essential, delineating all key phases from enrolment to information analysis and reporting. This timeline must be practical, considering the complexities of gathering and analyzing information while allowing for potential adjustments to the apparatus, which could arise from customer feedback or alterations in manufacturing processes. These adjustments, as emphasized by the UK MHRA's recent announcement, must be thoroughly documented to meet regulatory expectations and maintain patient safety.
In the context of real-world applications, as seen with Planatome's surgical blades, the post-market clinical follow-up plan should aim to capture data on clinical outcomes such as tissue damage, recovery times, and patient pain levels. This real-world evidence can then inform future product improvements and regulatory submissions.
When developing the plan for post-market clinical follow-up, it is crucial to take into account the nature of the inquiry, the reasoning behind the selected techniques, and information pertaining to the product and any reference devices. The plan should outline steps to minimize bias, including randomization, and detail the ethical considerations, especially when involving vulnerable subjects. According to Bijan Elahi, an experienced specialist in safety risk management for healthcare equipment, it is crucial to guarantee that the plan for Post-Market Clinical Follow-up (PMCF) covers all areas of risk management, including the design, compatibility, and operation of the components, as well as the potential effects on patient safety and equipment functionality.
In summary, a post-market clinical follow-up plan should be comprehensive and detailed, incorporating a clear definition of research questions, a well-characterized study population, precise sample size calculations, appropriate data collection methods, and a realistic study timeline. The ultimate goal is to safeguard patient outcomes while adhering to regulatory standards and responding to the evolving medical device landscape.
Implementing PMCF Activities
To efficiently conduct Post-Market Clinical Follow-up (PMCF) tasks, it is crucial to comply with a well-defined procedure. At first, the emphasis is on the careful gathering of clinical information, aligning with the pre-established PMCF plan and employing the selected methodologies for information acquisition. The next stage requires a thorough examination of the collected information, utilizing statistical techniques that are most suitable for the dataset.
Upon completion of the data analysis, a comprehensive risk assessment is conducted, drawing from the accumulated data to pinpoint any potential safety or efficacy concerns. This risk assessment is vital for maintaining the highest standards of patient care. After conducting the risk assessment, it is crucial to provide thorough and accurate reporting that encompasses the findings of the post-market clinical follow-up in a comprehensive and precise manner.
The last, yet ongoing, stage of post-market clinical follow-up is the incessant improvement of the procedure. This involves a regular review and critical evaluation of the procedures, identifying opportunities for improvement and implementing the necessary modifications to optimize the process. This iterative approach not only ensures compliance with regulatory standards but also fosters innovation, ultimately contributing to the elevation of patient care and safety.

Evaluating and Reporting PMCF Data
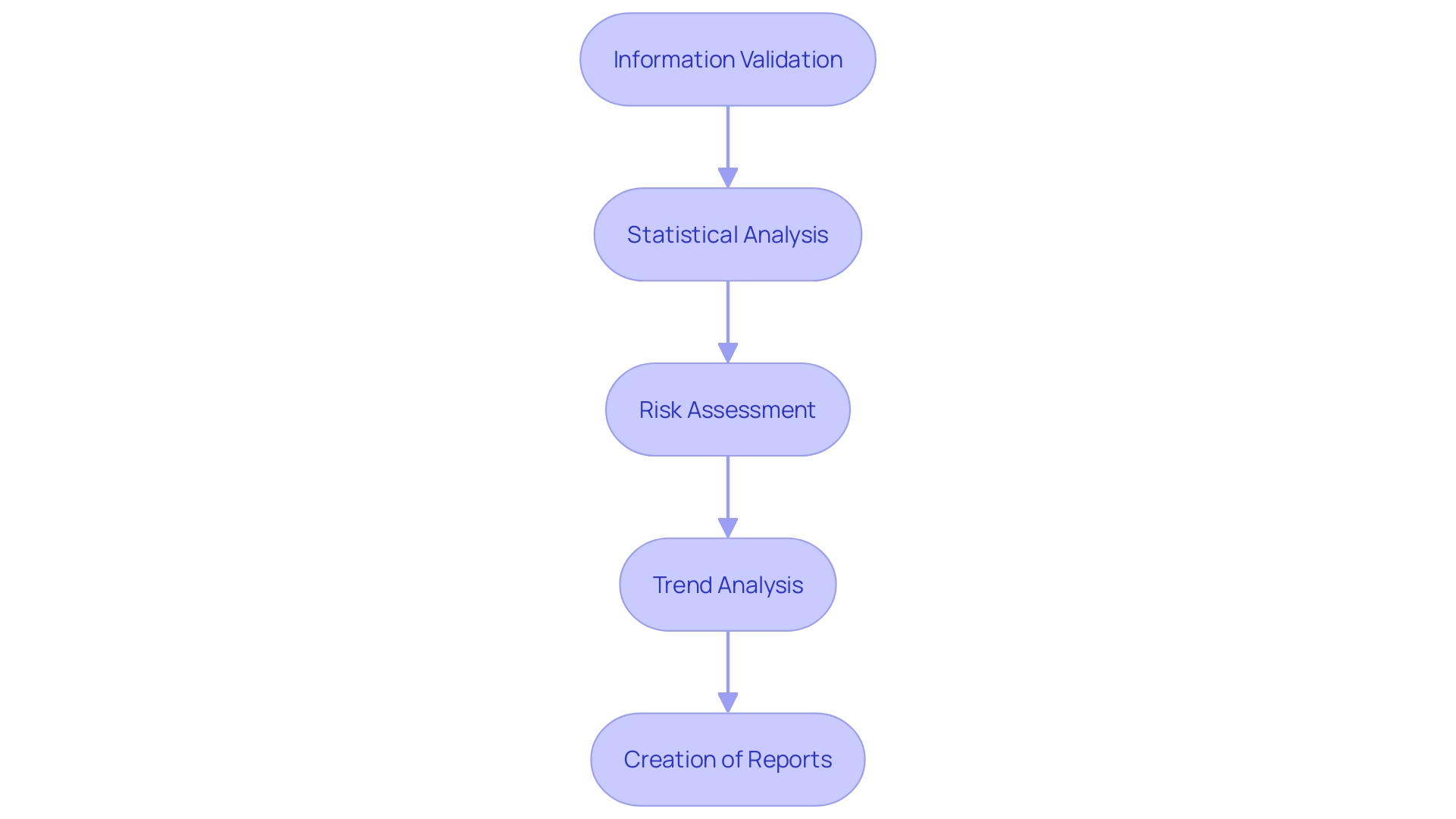
The post-market clinical follow-up is a systematic and proactive procedure to continuously monitor the safety and effectiveness of a medical instrument throughout its anticipated lifespan. The evaluation and reporting of PMCF involve a series of critical steps that ensure the ongoing protection of patient health and the optimization of device performance. These steps are:
-
Information Validation: The initial stage requires thorough validation of the gathered information for accuracy, completeness, and reliability. This is essential to ensure that the subsequent analysis is based on solid and trustworthy information.
-
Statistical Analysis: Once the information is validated, it undergoes statistical analysis. This is a crucial step where advanced statistical methods are used to interpret the information, which can include survival curves, line graphs, or bar graphs with complex features. The objective is to draw significant conclusions that can inform clinical practice and instrument development.
-
Performing a thorough risk assessment using post-market clinical follow-up information is crucial to evaluate the safety and efficacy of the product. This analysis helps identify any potential risks associated with the equipment and forms the basis for any necessary corrective actions.
-
Trend Analysis includes the recognition and examination of trends or patterns within the data. Recognizing these trends is vital for predicting and mitigating future risks, ensuring the device continues to perform as intended and that patient safety is upheld.
-
The final step of the reporting process is the creation of transparent and succinct reports. These reports communicate the information, analysis, and important findings to stakeholders, such as regulatory bodies, healthcare professionals, and the scientific community.
The significance of efficient data management and reporting in post-market clinical follow-up cannot be emphasized enough, as it contributes to the ongoing enhancement of healthcare tools and helps uphold public confidence in these vital instruments. With the World Health Organization estimating two million diverse types of healthcare devices on the market, the influence of comprehensive post-market clinical follow-up is extensive, impacting the quality and safety of patient care worldwide. As we transition into a period of digital health and advanced medical technologies, incorporating strong post-market clinical follow-up (PMCF) procedures is increasingly vital to foster innovation, guarantee adherence to regulatory standards, and ultimately improve patient outcomes.
Challenges and Best Practices in PMCF
Achieving success in Post-Market Clinical Follow-up (PMCF) requires a multifaceted approach to navigate its inherent complexities. One of the main obstacles lies in information collection, where the goal is to gather clinical information both accurately and in a timely manner. This information frequently originates from a wide range of sources, which requires a strong system for effective and safe information exchange. Real-time information feeds and advanced digital technologies can play a significant role in enhancing the ability to support PMCF activities and ensure that the information collected is reflective of the community's health.
When it comes to analysis, the use of suitable statistical techniques on intricate sets is essential. Considering the estimation made by the World Health Organization (WHO) that there exist two million distinct types of healthcare tools available for sale, each with the potential to affect millions of patients, the significance of precise data analysis cannot be emphasized enough. The insights gathered from such analysis inform continuous improvements and adherence to regulatory standards.
Allocation of resources is an additional challenge, as plans for post-market clinical follow-up must be supported by sufficient personnel and budgetary considerations. This is where strategic planning and the understanding of current and future industry trends become invaluable. As emphasized by the Who's description of a healthcare apparatus, the influence on patient quality of life is significant, making the effective distribution of resources a crucial element of post-market clinical follow-up.
Navigating regulatory compliance is another key aspect, with the health sector constantly evolving to align with ambitious goals like those set out by the Paris Agreement. Regulatory compliance is not just about adhering to current standards but also about anticipating changes and adapting accordingly. This proactive stance ensures that medical devices continue to provide the intended benefits without compromising patient safety.
To address these challenges, best practices are pivotal. Cooperation among stakeholders, such as manufacturers, healthcare professionals, and regulatory authorities, is crucial for a unified post-market clinical follow-up process. Continuous monitoring and evaluation help in identifying and addressing gaps promptly, while comprehensive documentation ensures transparency and traceability. Lastly, staying abreast of the latest regulations and industry best practices is a form of continuous learning that enhances the effectiveness and efficiency of PMCF activities.
Importantly, the joint endeavors of leading statisticians from various nations, as observed at the UN Statistical Commission, have highlighted the importance of such involvement and the utilization of citizen information in regulation of healthcare instruments. The push for digitalization in the medical device sector, as highlighted by industry experts, reinforces the importance of leveraging data effectively for the advancement of medical device safety and effectiveness.
Conclusion
In conclusion, Post-Market Clinical Follow-up (PMCF) is crucial for ensuring the safety and efficacy of medical devices. It involves continuous data gathering, monitoring, and assessment post-market release. PMCF activities play a vital role in preventing device-related complications and supporting regulatory compliance.
The key objectives of PMCF include long-term observation of device performance, detection and evaluation of adverse events, and accumulation of additional clinical evidence. By leveraging diverse methods such as passive and active surveillance systems, PMCF utilizes real-world data from registries, electronic health records, and administrative databases.
A robust PMCF plan is essential for maintaining patient care and regulatory compliance. This plan should encompass strategic data collection, comprehensive statistical methodology, clear timelines, and appropriate resource allocation. It also needs to anticipate future standards and technological advancements to drive innovation in medical devices.
Designing an effective PMCF system involves defining precise objectives, identifying relevant clinical data sources, selecting appropriate collection tools, and adhering to ethical considerations. Integration of service design principles and smart digitalization strategies is crucial in today's healthcare landscape.
Evaluating and reporting PMCF data involves steps like data validation, statistical analysis, risk analysis, trend analysis, and clear reporting. Effective data management and reporting contribute to continuous improvement in medical devices and maintain public trust.
Challenges in PMCF include data collection, analysis, resource allocation, and regulatory compliance. Best practices involve stakeholder collaboration, continuous monitoring and evaluation, comprehensive documentation, and staying updated with regulations and industry standards.
In summary, PMCF is essential for promoting innovation, enhancing safety, and ensuring global harmonization in medical device standards. It supports patient outcomes, regulatory compliance, and responds to the evolving medical device landscape. By implementing PMCF activities effectively, we can continuously improve medical devices and enhance patient care globally.




