Introduction
Serious Adverse Events (SAEs) and Suspected Unexpected Serious Adverse Reactions (SUSARs) are critical aspects of clinical research that require accurate identification, reporting, and management. Understanding the definitions of SAEs and SUSARs is essential in maintaining vigilance and ensuring the safety of patients in clinical trials. These definitions guide the reporting process and are comparable to the Vaccine Adverse Event Reporting System (VAERS) in monitoring vaccine safety.
Both regulatory authorities, such as the FDA, and pharmaceutical companies like Pfizer emphasize the importance of clear definitions and robust reporting mechanisms to uphold transparency and patient well-being. This article explores the responsibilities of key stakeholders, the reporting processes, the timeline for reporting SUSARs, and the significance of reporting to ethics committees and regulatory bodies. It also highlights the assessment and documentation of SAEs, safety reporting requirements, urgent safety measures, and best practices for SAE reporting and compliance.
By adhering to these practices and guidelines, the clinical research community can ensure the highest standards of participant safety and the advancement of medical knowledge.
Definition of Serious Adverse Events (SAEs) and SUSARs
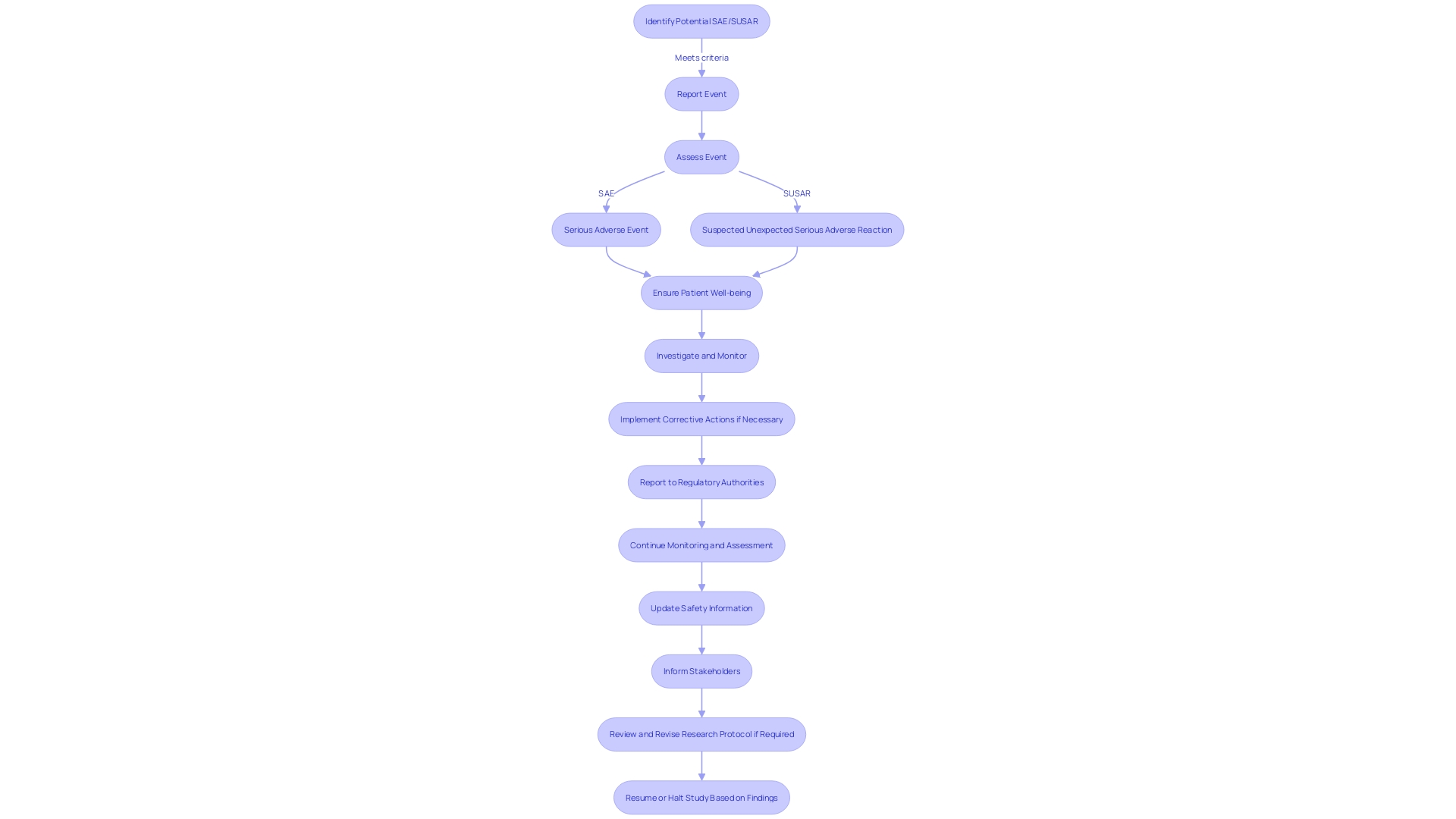
Grasping the intricacies of Serious Adverse Events (SAEs) and Suspected Unexpected Serious Adverse Reactions (SUSARs) is essential for their precise identification, reporting, and handling in research. An SAE encompasses any untoward medical occurrence that can lead to death, poses a threat to a patient's life, necessitates hospital admission or prolongs a current hospital stay, results in persistent or significant disability/incapacity, or is linked to a birth defect or congenital anomaly. In contrast, SUSARs are SAEs that are not anticipated given the characteristics of the medicinal product being investigated or the patient demographics.
The significance of these definitions cannot be underestimated, as they direct the vigilance within the field of clinical trials, much like the Vaccine Adverse Event Reporting System (VAERS) has done within the realm of vaccine well-being. VAERS, praised for its effectiveness in identifying concerns about well-being, exemplifies the crucial role of systematic reporting in maintaining public health and strengthening the well-being of medical interventions.
The FDA, a guardian of public health, publishes rules to ensure that the reporting of adverse events is done in a manner that is both understandable and accessible to the general public, highlighting the agency's commitment to transparency and security. Pfizer's approach to safety reporting echoes this sentiment; by encouraging the reporting of adverse events, the pharmaceutical giant aims to maintain a meticulous record of the safety profile of its medicines. This joint endeavor, observed in diverse institutions and systems, emphasizes the importance of precise definitions and strong reporting mechanisms in protecting patient well-being in medical studies and beyond.
Responsibilities in SAE Reporting
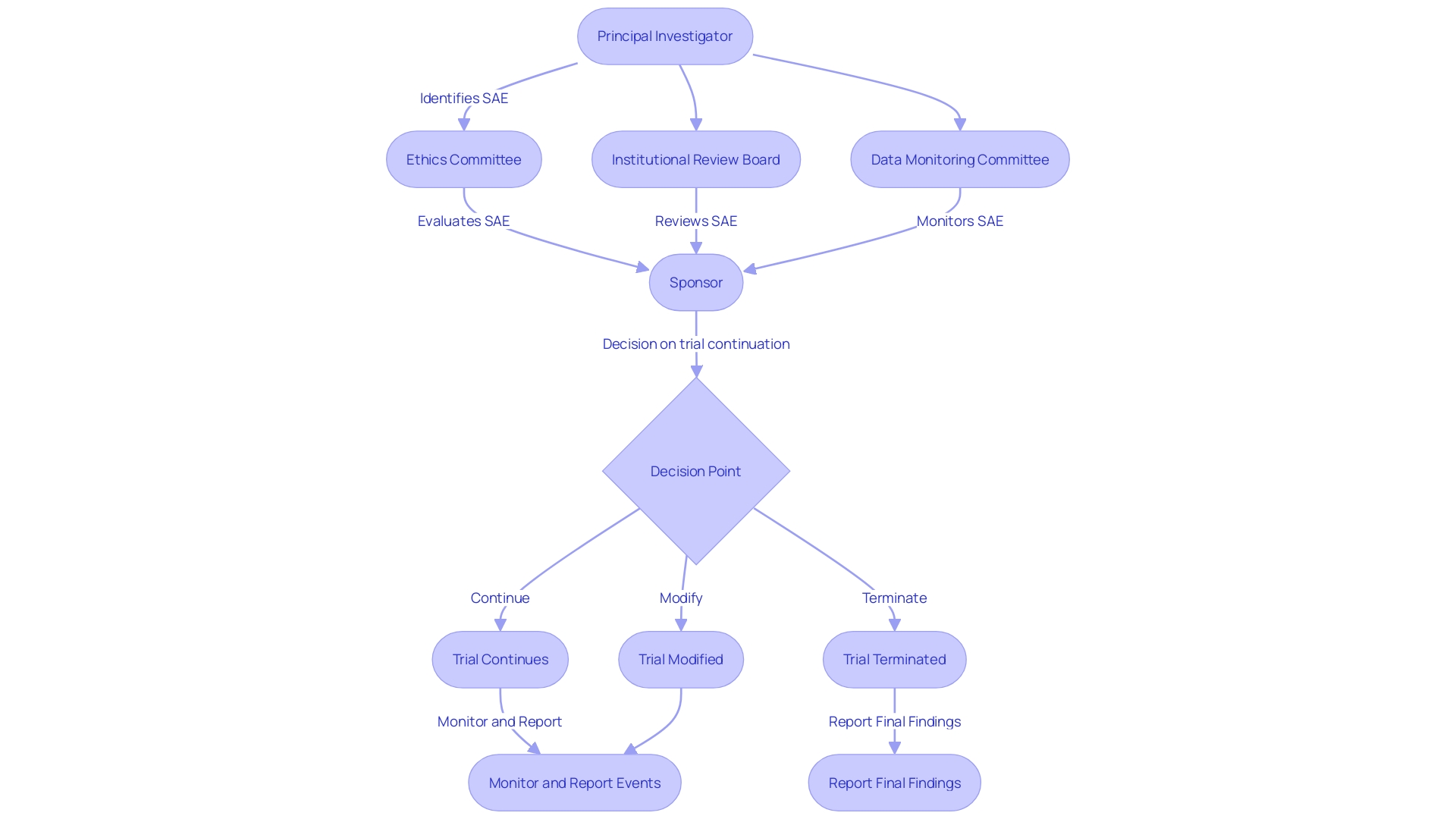
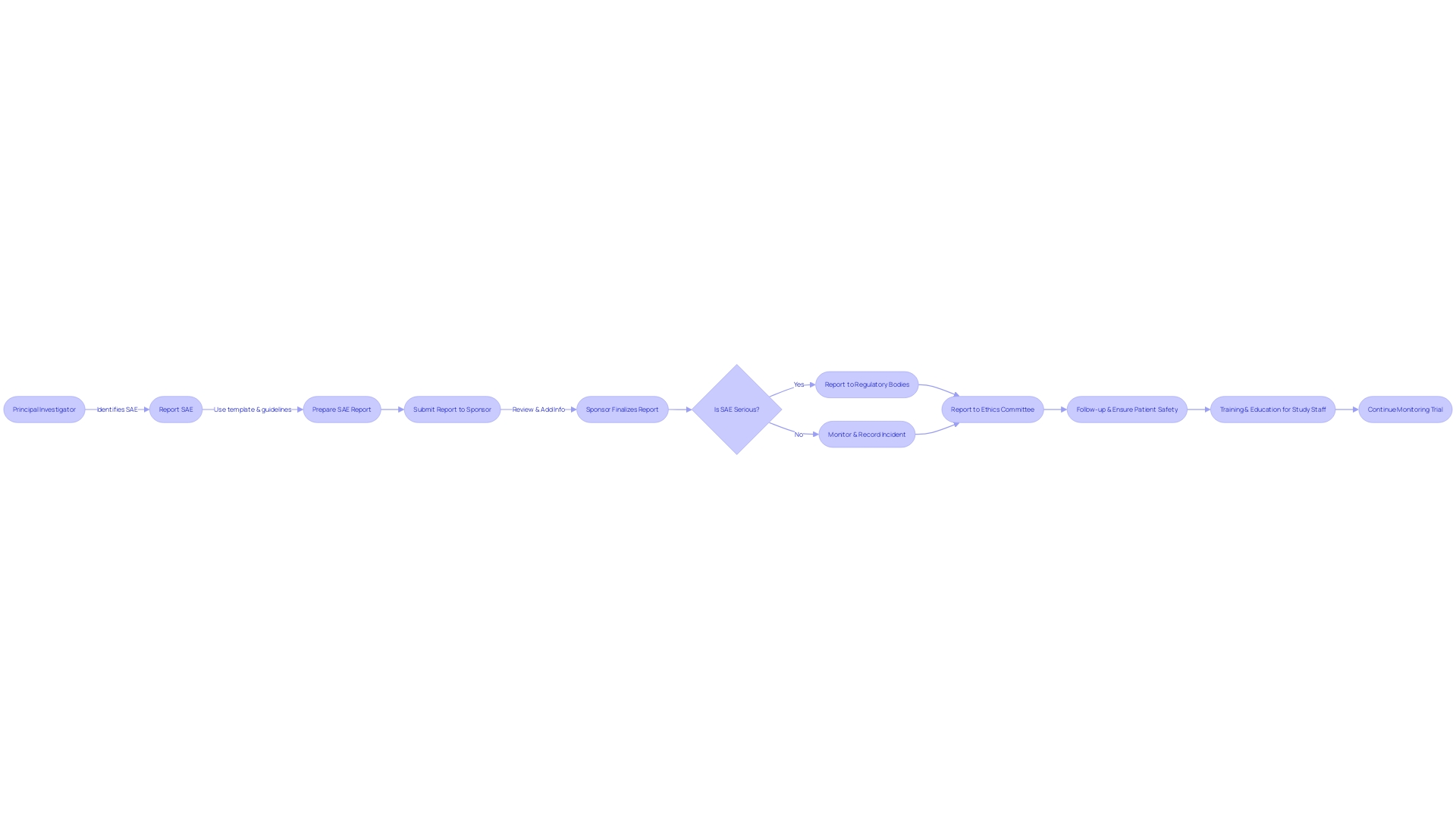
Efficient Serious Adverse Event (SAE) reporting in clinical experiments is a collaborative effort involving multiple key players, each with defined roles that are crucial for the protection of study participants and the integrity of the research. The Principal Investigator (PI) bears the crucial responsibility of protecting participant well-being, which involves promptly reporting any serious adverse events (SAEs) that may arise during the study. The sponsor, such as a pharmaceutical company or a Contract Research Organization (CRO), is responsible for supervising the trial's conduct and ensuring that SAE reports are submitted to regulatory authorities without delay.
Ethics committees and Institutional Review Boards (IRBs) play an oversight role, evaluating SAE reports to confirm the protection of participant well-being and the maintenance of study integrity. In this complex procedure, the recent preliminary advice from the FDA highlights the significance of Data Monitoring Committees (DMCs) in overseeing study well-being, reflecting a continuous dedication to improving the excellence of medical investigation by outlining protocols for their operation.
The importance of these roles is further highlighted by the European good practice (GCP) guidance, which includes a new section on data governance. This guidance, emphasized at the Outsourcing in Clinical Trials (OCT) Europe 2024 conference, emphasizes that experiments must prioritize participant safety and the reliability of results, encouraging a thorough evaluation of scientific objectives against potential risks.
SAE reporting is not just about regulatory compliance; it is a fundamental component of ethical research practices. According to experts such as Gamertsfelder and Osipenko, experiments conducted on individuals can have a significant individual influence, as they undergo intrusive examinations and possible consequences with the aspiration of aiding in the progress of medical breakthroughs that could be advantageous for subsequent generations. This context of human experience underscores the gravity of SAE reporting, reminding us that behind every report are individuals whose well-being is paramount.
Therefore, comprehending the separate yet interconnected obligations of every stakeholder is crucial to guarantee that SAE reporting is carried out with the highest diligence, accuracy, and ethical consideration, reflecting an industry-wide commitment to participant well-being and the progress of medical knowledge.
Reporting Processes for SAEs and SUSARs
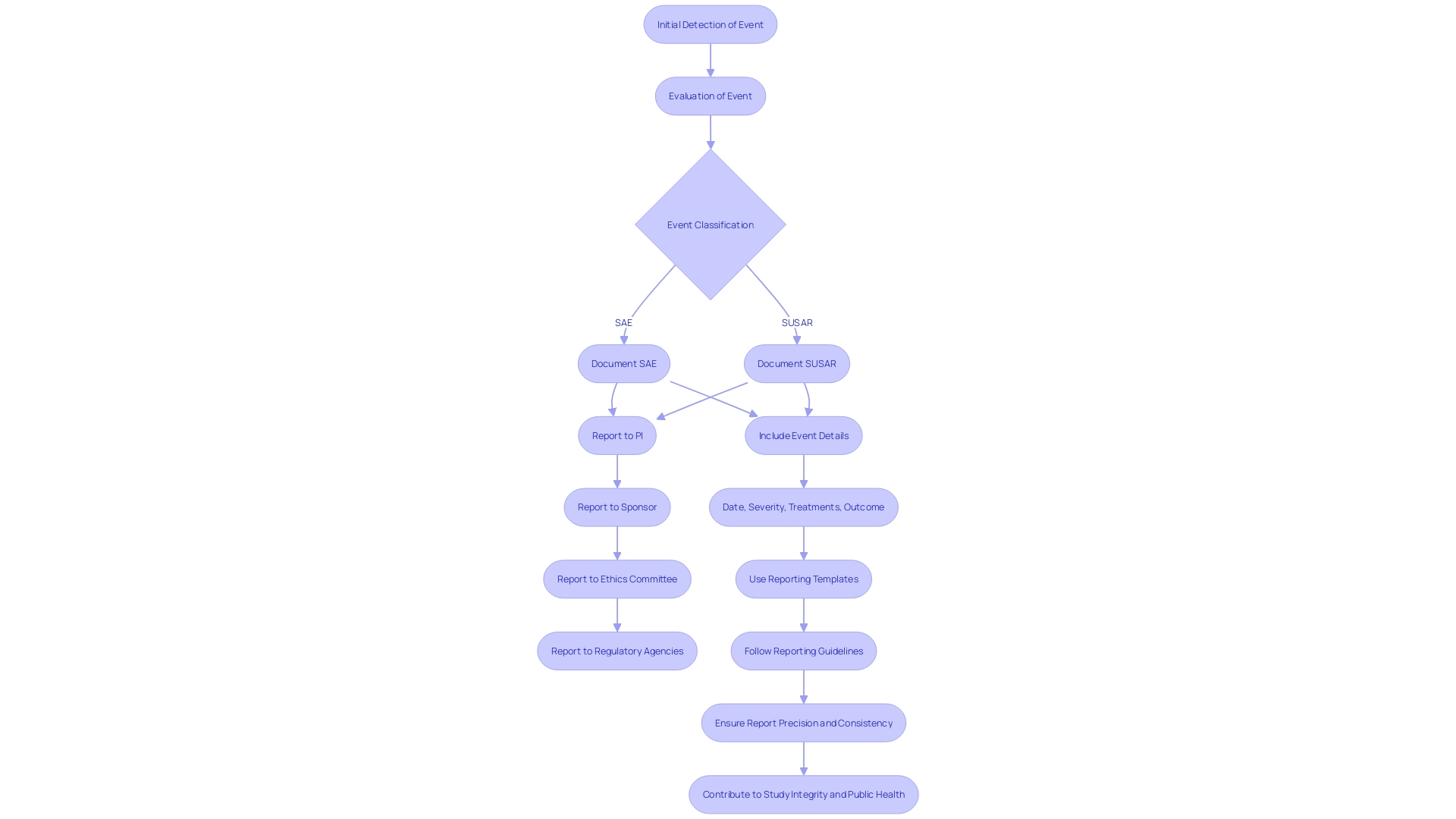
The thorough process of documenting Serious Adverse Events (SAEs) and Suspected Unexpected Serious Adverse Reactions (SUSARs) is crucial for the integrity of medical studies. It commences with the initial detection and evaluation of the event, leading to its classification as an SAE or SUSAR. Upon classification, meticulous documentation and prompt reporting to key stakeholders, such as Principal Investigators (PIs), sponsors, ethics committees, and regulatory agencies are imperative. Reports must encapsulate comprehensive details including the event's date, severity, associated treatments or interventions, and outcome. Adherence to standardized reporting templates and guidelines ensures the consistency and precision of these reports.
Particularly, the FDA's commitment to safeguarding public health underscores the necessity for clear, conspicuous, and neutral presentation of major statements in drug advertisements, a principle equally vital in the reporting of SAEs and SUSARs. This ethos is echoed in the agency's recent final rule emphasizing consumer-friendly language and dual modality in TV format ads, ensuring the major statement's readability and audibility. As emphasized by VAERS's effectiveness in vaccine monitoring, and the FDA's guidance on the beneficial role of Data Monitoring Committees (DMCs), these meticulous reporting practices are not only regulatory obligations but also contribute significantly to the broader public health landscape.
Timeline for Reporting SUSARs
Ensuring the well-being of participants and upholding the integrity of the research is a complex yet crucial aspect in the management of serious adverse events (SAEs) during trials. It is essential that SAEs, particularly suspected unexpected serious adverse reactions (SUSARs), are reported promptly to fulfill ethical obligations and regulatory mandates. Typically, the reporting of SUSARs must occur within 7 to 15 days of the sponsor becoming aware of the event. This prompt measure facilitates the ongoing monitoring of the experiment and helps maintain compliance with the governing regulatory agencies.
The European Medicines Agency (EMA) has highlighted the significance of openness and availability of experiment data for patients, sponsors, and healthcare professionals. The EMA's enhancements to the Clinical Trials Information System (CTIS) public portal are designed to simplify processes for sponsors and increase the user-friendliness of the system. This action aims to enhance the spread of information concerning ongoing experiments, which is crucial for patient safety and the progress of medical understanding. Furthermore, the introduction of new transparency rules ensures that information about clinical trials, including Sales, is made available sooner and more efficiently to all stakeholders.
The dynamic nature of clinical studies requires an understanding of various research terms such as 'delayed start' and 'delayed onset', which refer to the timing of human subjects' involvement in a study. Familiarity with these terms is crucial as they carry implications for the application process and compliance with regulatory requirements. A 'delayed start' indicates that the human subjects' investigation will begin later in the funding period with all necessary details included in the application. In contrast, 'delayed onset' indicates that the investigation plans involving human subjects are not fully defined at the time of application and require initial findings to finalize.
Furthermore, the function of medical investigation goes beyond the experiment in question, as it frequently includes individuals who, despite their own health obstacles, contribute to forthcoming medical progress. Their involvement is evidence of the selfless nature of medical studies, with the expectation that their participation might benefit future generations. Ensuring the timely and accurate reporting of SUSARs honors the contributions of these individuals by upholding the highest standards of safety and academic integrity.
In conclusion, the rigorous and timely reporting of SUSARs is imperative for the ethical conduct of trials. The recent updates by regulatory bodies like the EMA, alongside a clear understanding of investigation terminology, contribute to a more efficient and transparent investigation environment. The collective efforts of all parties involved in clinical research are essential in advancing medical knowledge and ultimately improving patient outcomes.
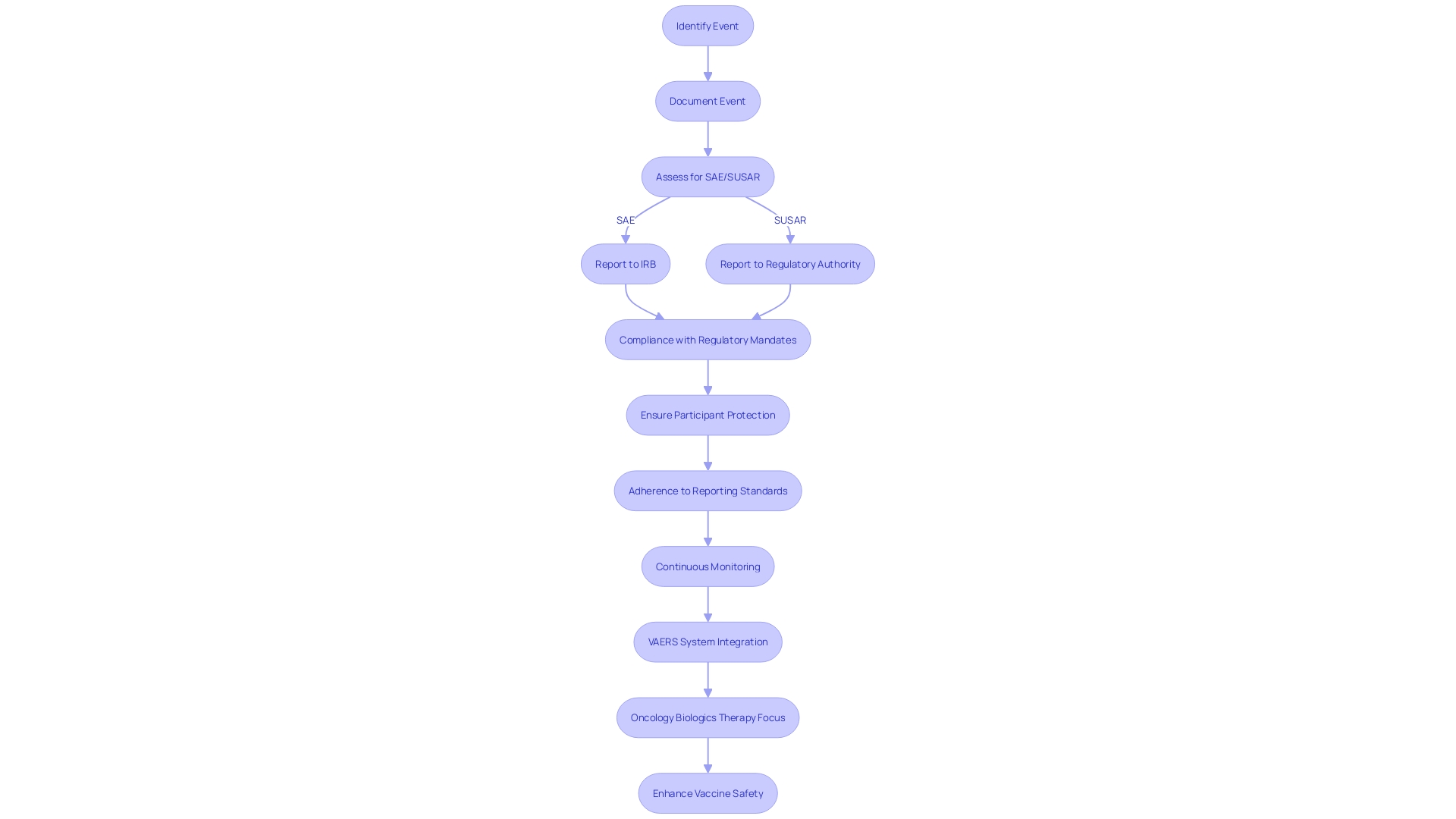
Reporting to the IRB and Regulatory Bodies
The complex nature of Serious Adverse Events (SAE) and Suspected Unexpected Serious Adverse Reactions (SUSARs) reporting is vital for the protection of study participants and compliance with regulatory mandates. Prompt and thorough communication with Institutional Review Boards (IRBs) is paramount when such events occur, as they serve as the ethical backbone, ensuring the rights and well-being of subjects are safeguarded. Concurrently, regulatory authorities, including the FDA, mandate precise reporting protocols. These encompass timely expedited or periodic updates, formatted in accordance with stringent guidelines. In the realm of biologics, where the market is burgeoning, particularly in oncology, the precision in reporting is even more critical. As biologics therapy for cancer is expected to be the main focus in the pharmaceutical industry with a projected market growth to 786 billion by 2029, the safety and effectiveness data generated from medical studies become a cornerstone for the ongoing progress and approval of these intricate therapeutics.
Adherence to reporting standards is not just a regulatory obligation but also a moral duty, considering the altruistic contribution of thousands of experiment volunteers annually in the U.S., many of whom may not directly benefit from the investigational therapies they receive. It is the scientific knowledge accumulated from the trials that justify the risks undertaken by participants. This ethical framework is reinforced by FDA's recent initiatives to enhance the clarity and neutrality of drug advertisements, ensuring that the potential risks of new drugs are communicated effectively to the public. The FDA's Postmarketing Requirements and Commitments Searchable Database further demonstrates the agency's dedication to transparency by providing access to information on postmarketing requirements and commitments pertaining to the well-being and effectiveness of patients. The database, updated quarterly, is evidence of the dynamic nature of oversight in the field of medical investigation and the ongoing efforts to improve the processes that safeguard public health.
Assessment and Documentation of SAEs
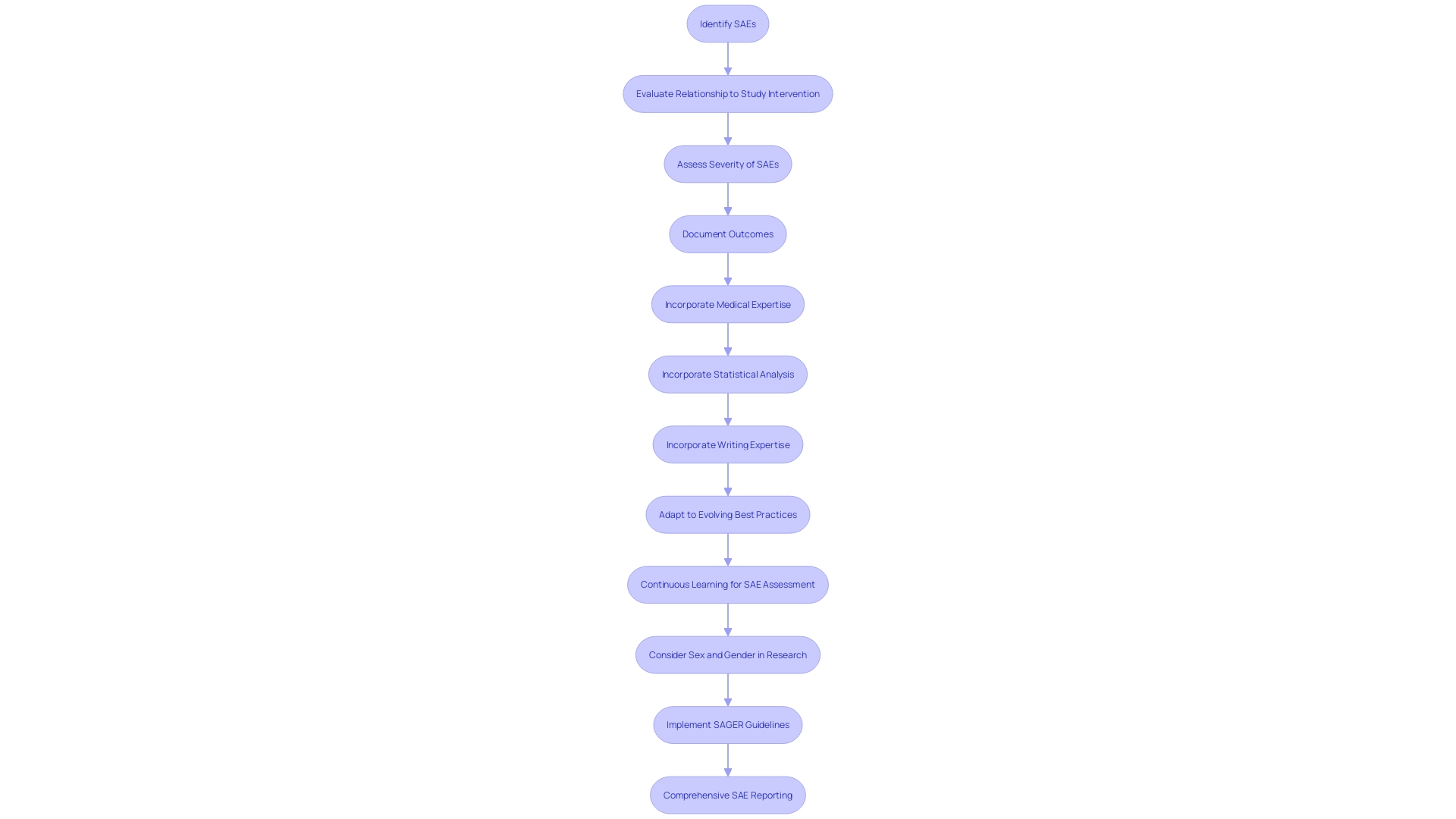
In clinical research, the thorough analysis of Serious Adverse Events (SAEs) is crucial to ensure patient well-being and maintain the integrity of the trial. The identification of an SAE triggers a detailed evaluation process to ascertain its relationship to the study intervention, its severity, and whether it was anticipated. This evaluation is meticulous, involving an extensive examination of pertinent data such as medical history, laboratory findings, and any other information related to the event. The outcomes of this evaluation must be meticulously recorded, with a focus on capturing all vital details. The clarity and precision of this documentation are vital, as it underpins the accuracy of SAE reporting and the subsequent data analysis. This process not only complies with regulatory requirements but also contributes to the collective understanding of the intervention's well-being profile. When creating these documents, it is essential to incorporate knowledge from different fields, such as medical, statistical, and writing expertise, to guarantee the development of comprehensive reports that adhere to the rigorous criteria established by regulatory agencies such as the FDA. Acknowledging the dynamic nature of safety analytics, it is also essential for professionals to engage in continuous learning to adapt to evolving best practices and methodologies in assessment.
Safety Reporting Requirements for Clinical Trials
Within the realm of clinical research, the attentiveness towards participant well-being is of utmost importance, requiring the regular submission of various well-being reports such as Development Update Reports (DSURs) and periodic well-being documents. These reports are not just administrative requirements; they encapsulate the complete security story of an investigational product, highlighting any emerging security concerns. It is through this meticulous process that regulatory compliance is upheld, ensuring the safety of participants is continually monitored throughout the trial's progression.
Under the watchful eye of regulatory bodies like the FDA and EMA, experiments involving patients are progressively embracing cutting-edge technologies such as AI and ML, guided by a 'risk-based approach' as recommended by the most recent EU AI Act. As these innovations hold potential for the healthcare industry, they also bring forth new compliance challenges, necessitating updated guidelines that shape the landscape for medical experiments. In light of this, the FDA has recently broadened the scope of its guidance to encompass disasters and public health emergencies at large, focusing on a risk-based evaluation for clinical trial sites and participant monitoring.
In the current climate, the FDA's role in ensuring the safety and efficacy of medical interventions is highlighted by its recent publication of the "Direct-to-Consumer Prescription Drug Advertisements" final rule. This rule establishes standards for presenting major side effects and contraindications in consumer-friendly language, ensuring clarity and neutrality in direct-to-consumer drug ads on television and radio.
The significance of monitoring for the purpose of ensuring the absence of hazards is further emphasized by the proposed guidance on the function and functioning of data monitoring committees (DMCs), which is awaiting finalization. Once in effect, this guidance will supplant the 2006 'Establishment and Operation of Clinical Trial Data Monitoring Committees' and aims to enhance trial oversight.
Moreover, experiments in the medical field, as fundamental structures of medical investigation, function throughout different stages, each with separate objectives ranging from evaluation of well-being in Phase one to assessment of effectiveness and well-being in subsequent phases. The well-being of patients remains a crucial concern throughout these phases, as shown in the case of elderly participants in an examination for transthyretin-mediated amyloidosis. These individuals, while managing multiple health conditions, contribute to the advancement of medicine, motivated by the hope that future generations might be spared their suffering.
In the end, the story of medical experiments is about finding a balance between progress and ensuring the well-being of patients, a process characterized by regulatory watchfulness, safeguarding participants, and the pursuit of advancements in healthcare.
Urgent Safety Measures and Amendments
The landscape of clinical research is constantly changing, and with it, the need for strong measures to protect the well-being of study participants. A recent schizophrenia trial, where assessments were made by site staff, underscores the importance of adapting to circumstances such as the COVID-19 pandemic and geopolitical events like the war in Ukraine, which necessitated a switch from in-person to phone visits. This adaptation, while necessary, highlighted variations in data quality and the need for flexible yet rigorous monitoring procedures.
Considering these challenges, regulatory agencies are increasing their attention on patient well-being amidst innovation. The most recent guidance from FDA for sponsors of studies outlines considerations for the establishment and operation of Data Monitoring Committees (DMCs), emphasizing the importance of independent oversight in research. These committees are essential to the implementation of urgent measures, acting as a safeguard to swiftly address unforeseen risks and ensure the integrity of trial outcomes.
The incorporation of artificial intelligence (AI) and machine learning (ML) in research further amplifies the intricacy of monitoring. Regulatory bodies, including the European Medicines Agency (EMA) and the European Union (EU), emphasize a 'risk-based approach' to AI usage, demanding transparency and careful oversight to navigate the delicate balance between innovation and patient safety.
Additionally, Dr. Jeremy Farrar, the Chief Scientist of the WHO, underscores the importance of diversity and fair access in clinical tests. Data from 2022 shows that less than 5% of experiments included pregnant women and only 13% included children, emphasizing the disparities in experiment participant demographics. This absence of diversity can influence the generalizability of experiment outcomes and the applicability of interventions across various populations.
The FDA's commitment to public health is reflected in their recent ruling on direct-to-consumer drug advertisements, ensuring that the major side effects and contraindications are presented in a clear, conspicuous, and neutral manner. This degree of openness is reflected in the need for transparent protocols in medical experiments, especially in instances where immediate precautionary actions, like temporary pauses in the study or adjustments to the procedure, are required.
While dealing with these challenges, the primary objective of the research community remains unchanged: giving priority to participant safety by implementing clear and effective procedures that adapt to the ever-changing nature of medical studies.
Best Practices for SAE Reporting and Compliance
To guarantee that Serious Adverse Events (SAEs) are reported with the utmost precision and effectiveness during medical experiments, it is crucial to embrace a set of optimal approaches. Key to this is the establishment of robust communication channels between the Principal Investigator (PI), the trial sponsor, ethics committees, and regulatory bodies. Utilization of standardized templates and guidelines for reporting can significantly enhance the accuracy and comprehensiveness of SAE documentation. Moreover, consistent training and educational initiatives for study staff can bolster their understanding and adherence to SAE reporting protocols. These practices not only strengthen adherence to regulatory standards, but they also enhance patient safety and contribute to the integrity and success of research.
Recent updates to the European good practice guidelines for medical studies highlight the significance of data management in medical trials. As emphasized by Silvia Perez at the Outsourcing in Clinical Trials (OCT) Europe 2024 conference, the upcoming guidance revisions focus on protecting participant's rights and wellbeing, ensuring study results' reliability, and emphasizing rigorous evaluation of the scientific objectives and associated risks. These considerations are fundamental to the design and execution of trials that meet the necessary standards for patient care and treatment outcomes. Furthermore, in the realm of regulatory guidelines, the inclusion of AI and ML technologies in clinical research is becoming increasingly prevalent. This innovation brings with it a heightened need for transparency and a 'risk-based approach' as suggested by the latest EU AI Act, ensuring that patient safety remains at the forefront amidst technological advances.
Conclusion
In conclusion, accurate identification, reporting, and management of Serious Adverse Events (SAEs) and Suspected Unexpected Serious Adverse Reactions (SUSARs) are crucial in clinical research. Clear definitions and robust reporting mechanisms, comparable to the Vaccine Adverse Event Reporting System (VAERS), ensure transparency and patient well-being. Collaboration among stakeholders, including the Principal Investigator, sponsor, ethics committees, and Institutional Review Boards, is essential for effective SAE reporting.
Timely reporting of SAEs and SUSARs, using standardized templates and guidelines, fulfills ethical obligations and regulatory mandates. Compliance with safety reporting requirements, such as Development Safety Update Reports (DSURs), maintains ongoing safety monitoring and regulatory compliance. Reporting to IRBs and regulatory bodies protects participants and ensures adherence to mandates.
Urgent safety measures and amendments adapt to evolving circumstances, with independent oversight through Data Monitoring Committees ensuring swift implementation and trial integrity. The integration of AI and ML technologies requires transparency and careful oversight to balance innovation and patient safety.
Best practices for SAE reporting and compliance involve robust communication, standardized templates, and consistent training for clinical study staff. Updates to European good clinical practice guidelines emphasize data governance and participant rights. Adherence to these practices upholds the highest standards of participant safety and advances medical knowledge.
In conclusion, accurate and timely reporting of SAEs and SUSARs, collaboration among stakeholders, compliance with reporting standards, and adherence to best practices create an efficient, transparent, and ethical research environment. By prioritizing patient safety and following these guidelines, the clinical research community can contribute to the advancement of medical knowledge and improve patient outcomes.




