Introduction
Navigating the intricate world of medical research, particularly in clinical trials, presents numerous challenges and ethical considerations. From logistical hurdles to participant compensation, the complexities involved require meticulous attention.
In this article, we explore how Clinical Research Organizations (CROs) have become pivotal in addressing these challenges and revolutionizing the landscape of medical research. We delve into the services offered by CROs, their impact on study design, participant recruitment, data management, and regulatory compliance.
Furthermore, we examine a case study highlighting the role of CRO consulting services in overcoming the obstacles faced by patients in accessing life-changing clinical trials. Lastly, we discuss best practices for implementing CRO partnerships to ensure efficient and effective collaborations. Join us as we explore the world of CROs and their contribution to the advancement of medical research.
The Problem: Challenges in Medical Research
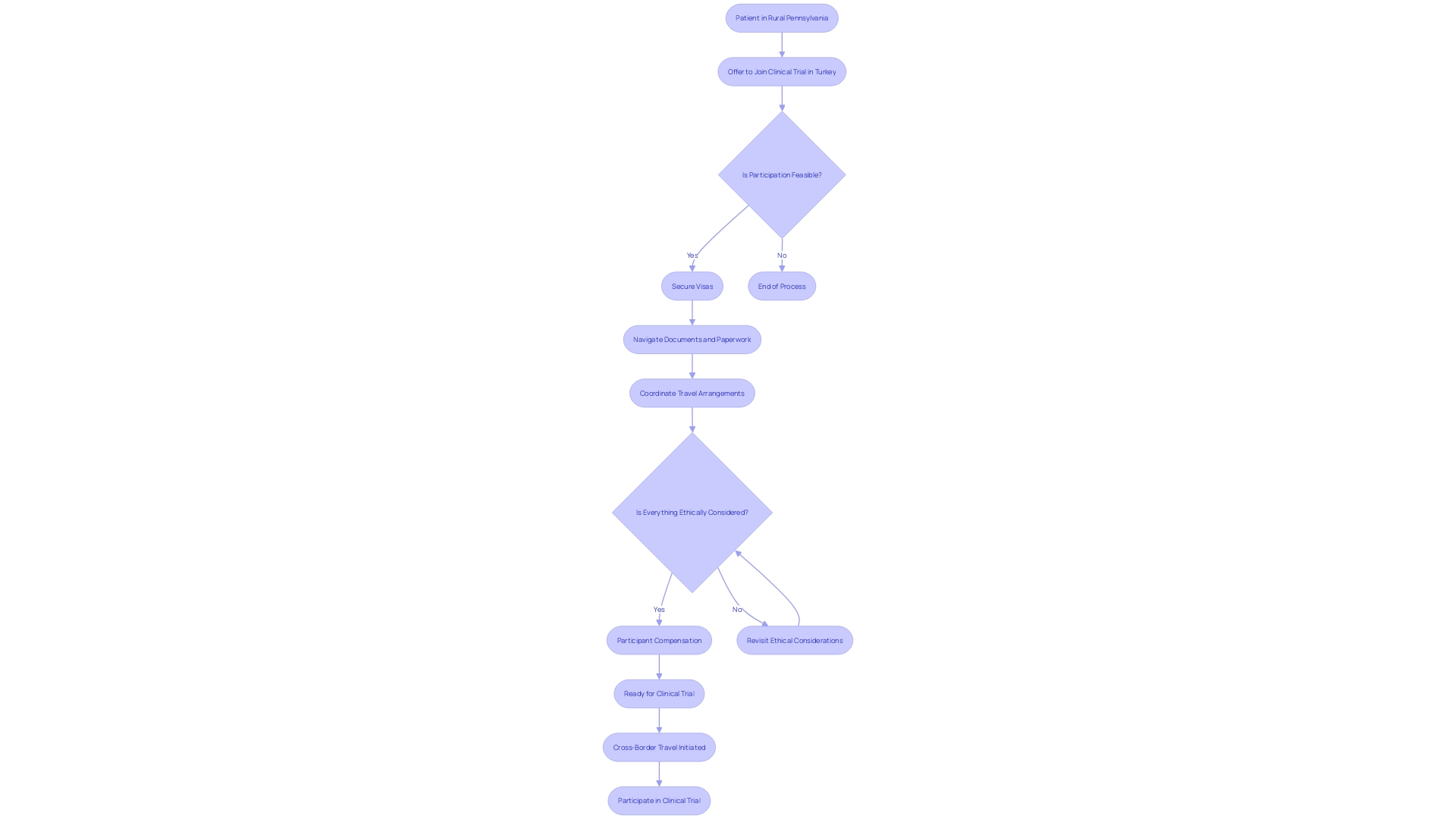
Navigating the procedures and challenges of medical research, particularly in clinical trials, presents a tapestry of logistical hurdles and ethical considerations that require meticulous attention. Take the example of a patient from rural Pennsylvania, afflicted with an ultra-rare disease devoid of FDA-approved treatments.
Presented with an opportunity to partake in a clinical study in Turkey, the patient and their family find themselves plunged into a whirlpool of cross-border travel complexities. The uncertainties abound: from securing visas to navigating unfamiliar paperwork, not to mention the coordination of travel arrangements in a foreign language environment.
Furthermore, when it comes to participant compensation, there's a marked shift in the ethical landscape. Historically viewed through a lens of caution to avoid unduly influencing participants' consent, there's an emergent focus on fair treatment and the rightful role of comprehensive reimbursement.
It’s becoming recognized that participants, like public service providers including first responders, deserve just compensation for their involvement, which often involves personal sacrifices, from covering research-associated expenses and dedicating time, to assuming participation risks and foregoing other opportunities. This real-world scenario underscores the pressing need for scrupulous planning and ethical considerations by clinical trial companies. In alignment with these intricate demands, insights from industry experts illustrate the pivotal role of rigorous study design and proactive decision-making. As elucidated by transaction advisory professionals, a considerable portion of these critical decisions, tied to a vast array of data points, are conceived years in advance and could benefit substantially from a more fortified and thoughtful approach in the early stages to optimize each phase of the clinical trial process.
The Solution: CRO Consulting Services
Clinical Research Organizations (CROs) have become pivotal in surmounting the myriad of challenges facing medical research today. The architecture of clinical trials is rooted in a well-articulated research question—a linchpin that holds together the study design, from the manipulation of variables to the measurement of outcomes. It's the meticulous comparative study between treatment and control groups that generate meaningful insights while rigorously mitigating biases.
Tailored participant recruitment is another forte of CROs. With rich networks and databases, CROs deploy targeted enrollment strategies, expediting the search for eligible candidates. As illustrated by the rural Pennsylvanian patient facing a daunting overseas clinical trial in Turkey, CROss are tasked with alleviating the logistical perplexities associated with cross-border travel in clinical trials, making life-changing trials more accessible.
A crux of contemporary research evolution lies in data handling—CROss harness advanced technologies for data management and leveraged analysis, ensuring precision and trustworthiness of trial data. Moreover, they bring to the table vast experience in threading through the intricate regulatory terrain, offering essential guidance that ranges from compliance to ethical standards adherence, rooted in a profound understanding of the legal and social ramifications of emerging technologies. Efficiency in CRO operations lends itself to optimal cost and temporal expenditure in trials.
By refining workflows and standardizing processes, CROss aim to eliminate redundancy and avert delays. Such prudence in planning is echoed in the insight from an advisory perspective—80% of trial-related decisions, often made years in advance, could yield improved outcomes if fortified by more in-depth, strategic planning from CROs. Overall, CROs offer an orchestration of services that not only address the manifold obstacles in medical research but also evolve the landscape for governance of emerging technologies, drawing from cross-sectoral lessons and enriching the governance ecosystem within the domain of health and medicine.
Case Study: The Impact of CRO Consulting Services on Medical Research
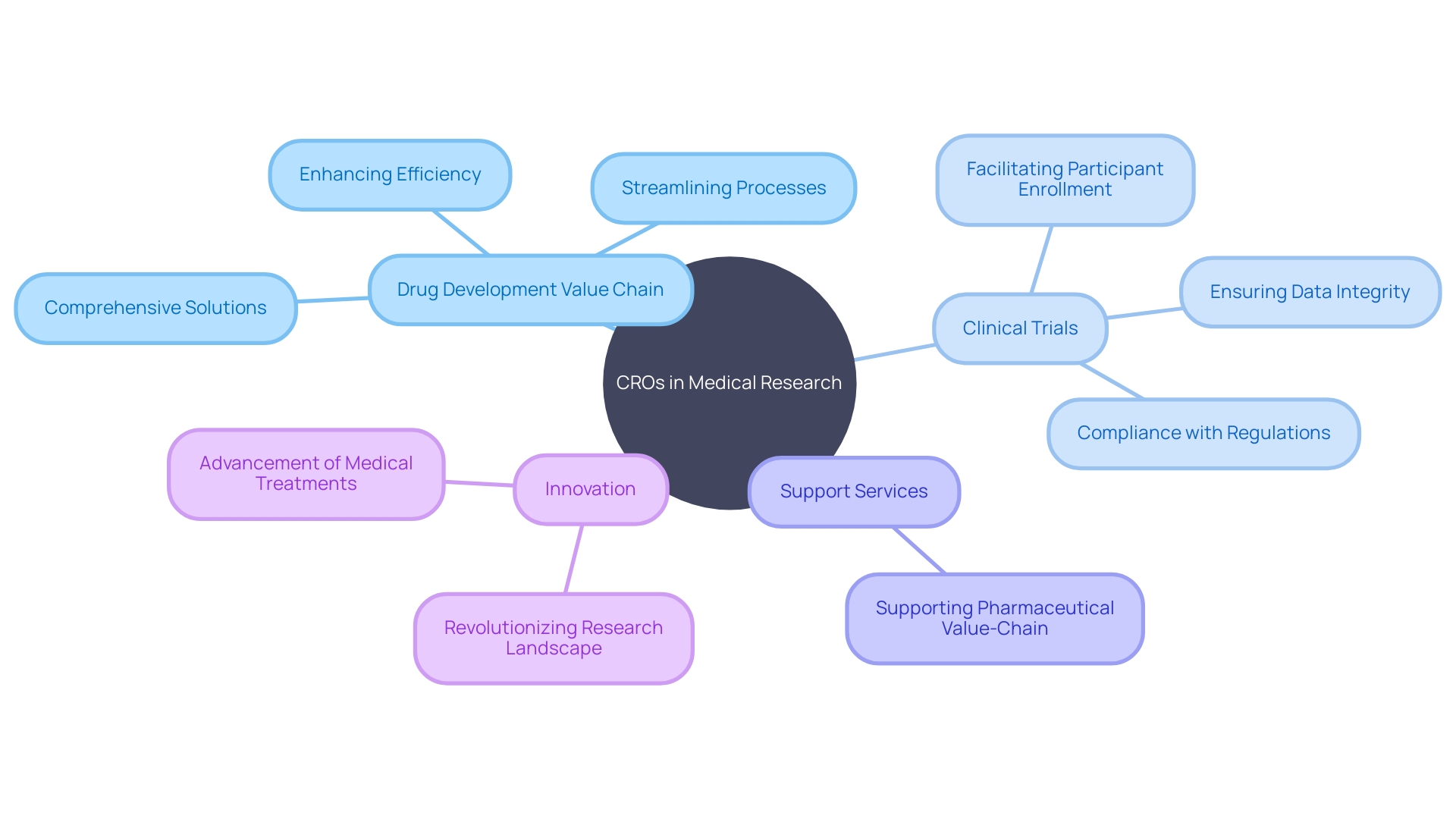
Contract Research Organizations (CROs) like CMIC Group have been revolutionizing the landscape of medical research by providing comprehensive solutions throughout the drug development value chain. Take, for instance, the difficulties encountered when a patient from rural Pennsylvania with an ultra-rare disease faces the possibility of traveling to Turkey for a clinical trial.
It's a daunting proposition, considering the myriad of logistical challenges, such as securing a visa, navigating foreign paperwork, and coordinating travel. Such scenarios underline the need for efficient and patient-centered solutions provided by CROss to navigate the multifaceted challenges of clinical trials.
This is where CROss step in with their extensive resources, domain expertise, and established networks to streamline processes and enhance the efficiency of medical research. CMIC, Japan's pioneer and leading CRO, with over three decades in the industry, offers an example of these capabilities.
They leverage their robust participant database and refined recruitment strategies to address the common challenge of timely participant enrollment faced by many pharmaceutical companies. By deploying advanced data management systems, CROss like CMIC facilitate precise and swift data collection and analysis, leading to heightened data integrity and compliance with regulatory mandates. The advantages of these end-to-end solutions are palpable. Pharmaceutical companies often observe a marked increase in recruitment efficiency, a reduction in trial durations, and cost savings. Moreover, the support CROss offer extends far beyond the clinical phase, enveloping the entire pharmaceutical value-chain, which further exemplifies their role in fostering the successful advancement of medical treatments.
Results: Improved Efficiency and Accuracy in Medical Research
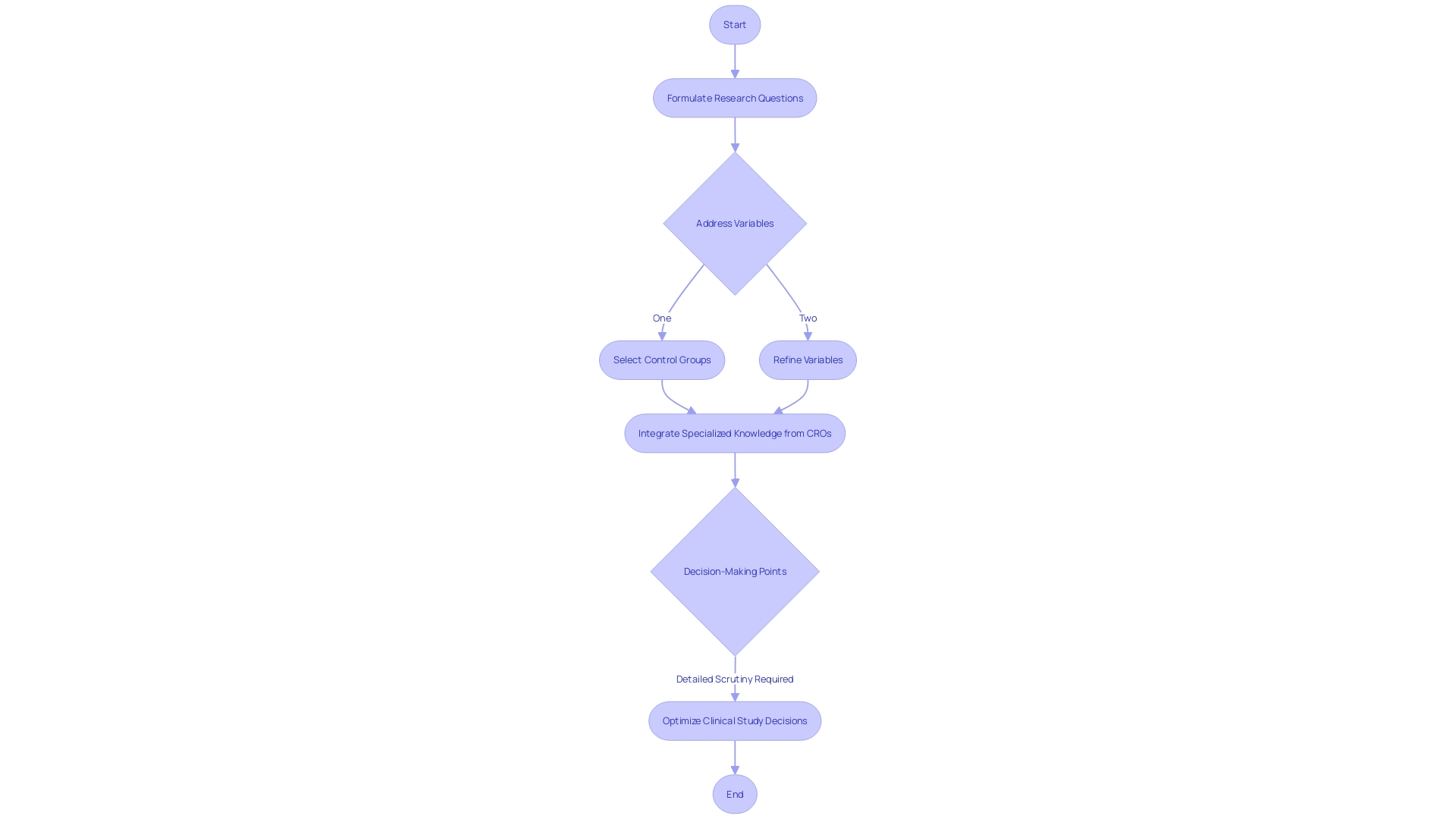
Advanced clinical study design and its impact on medical research cannot be overstated. At the core lies a thoroughly articulated research question, driving the precise engineering of how each variable will be addressed.
The selection of a suitable control group also proves critical in producing credible outcomes. CROss provide comprehensive support in this intricate formulation, integrating their specialized knowledge to enable finely tuned comparisons between treatment and control cohorts, thereby enhancing the study's validity.
Ever-increasing complexities in regulatory landscapes demand that researchers seek expertise from CROs, who diligently safeguard against non-compliance risks stemming from evolving protocols of good clinical practice and ethical standards. Treehill Partners reveal an industry insight where hindsight regularly illuminates the benefits of thorough preparation, suggesting 80% of previous clinical study decisions could have been optimized with detailed scrutiny.
Moreover, the CRO's role extends beyond study walls. Evaluating Treehill's services sheds light on the often-unseen logistical hurdles faced by patients, as in the case of a Pennsylvania resident navigating international travel to join a trial in Turkey.
The daunting checklist of managing visas, language barriers, and travel coordination underscores the breadth of CRO responsibilities. Finally, CMIC Group of Japan, a pioneer in the CRO arena, encapsulates the multifaceted role of these organizations. With over three decades of experience, they boldly assume responsibilities across the pharmaceutical value-chain, emphasizing customer-centric solutions that align with the various phases of drug development. A glimpse into their expansive offerings indicates their integral role in streamlining the journey from conception to market. Their bespoke services mirror the industry's progression, affirming the indispensability of cross in medical research advancement.
Lessons Learned: Best Practices for Implementing CRO Consulting Services
Efficient and effective practices in managing CRO consulting services are paramount within medical research. For impactful CRO partnerships, there are strategic methods that are instrumental to thriving collaborations:
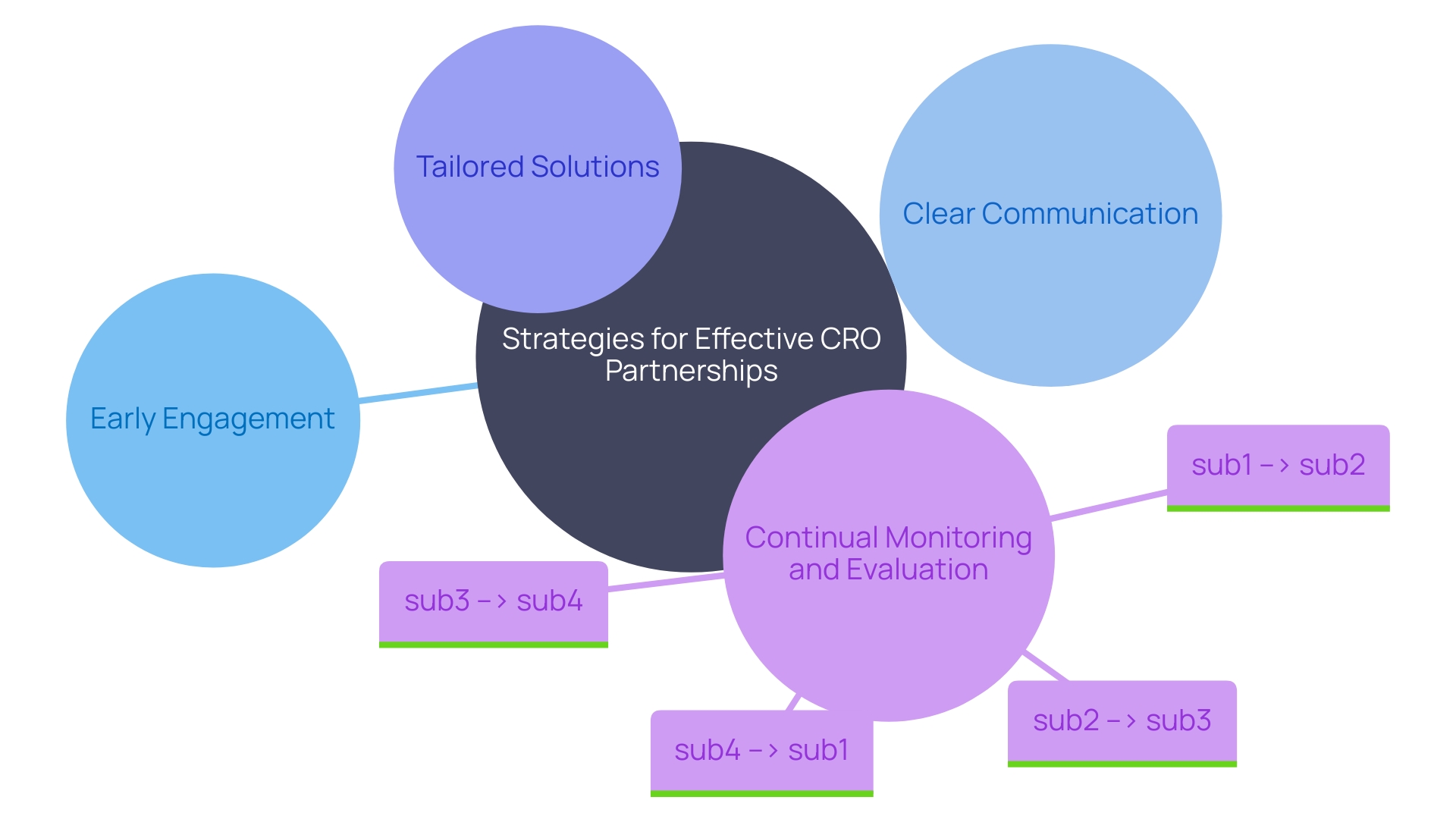
- Early Engagement: Launching CRO partnerships preemptively within the research lifecycle brings their proficiency to the forefront, particularly during study design, shaping protocols, and navigating regulatory landscapes.
This foundational approach sets the precedent for methodical clinical trial progress. 2. Clear Communication: Unambiguous dialogue with the CRO stands as a critical vein throughout the project.
It's essential to articulate objectives, timelines, and projected outcomes from the outset. Such precision in communication guards against potential disparities and nurtures a synergistic work environment. 3.
Tailored Solutions: Constructing bespoke solutions alongside the CRO is vital to surmount specific project hurdles. The goal is a consulting service that resonates with the distinct demands of each research study, mirroring the meticulous nature of inquiries into areas such as maternal-child health to distill nuanced insights from vast pools of data. 4.
Continual Monitoring and Evaluation: By keeping a vigilant eye on CRO performance metrics like recruitment efficiency and data integrity, and ensuring rigorous standards in ethics and regulatory adherence, projects can swiftly pivot to address concerns. This scrutiny is akin to the attention required when assessing the feasibility of patient participation in remote clinical trials, understanding the intricate logistics and considerations borne by patients and their families. Incorporating these best practices, researchers and pharmaceutical firms foster an elevated use of CRO services, driving forward the frontiers of medical research with precision and purpose.
Conclusion
In conclusion, Clinical Research Organizations (CROs) have revolutionized medical research by addressing its challenges and complexities. They excel in study design, participant recruitment, data management, and regulatory compliance.
CROs play a crucial role in study design, ensuring rigorous comparative studies and meaningful insights while mitigating biases. Their expertise in participant recruitment makes life-changing trials more accessible.
CROs excel in data management, utilizing advanced technologies for precise and trustworthy trial data. They navigate the regulatory landscape and provide guidance on compliance and ethical standards.
Efficiency is key in CRO operations, leading to optimal cost and time expenditure in trials. By refining workflows and standardizing processes, CROs enhance efficiency in medical research.
A case study with CMIC Group, a pioneer CRO in Japan, showcased their streamlined processes resulting in increased recruitment efficiency, reduced trial durations, and cost savings. Successful collaborations with CROs require best practices such as early engagement, clear communication, tailored solutions, and continual monitoring and evaluation. In summary, CROs are pivotal in advancing medical research. They optimize the research process, improve efficiency and accuracy, and foster the successful development of medical treatments. CROs are indispensable partners in the quest for medical research advancement.




