Introduction
The FDA's Breakthrough Devices Program is a pivotal initiative aimed at fast-tracking the development and review of innovative medical devices that can significantly improve treatment or diagnosis for life-threatening or irreversibly debilitating diseases. This program supports devices that offer new solutions for patients with no other treatment options, or that provide significant advantages over existing alternatives. With over 800 devices granted Breakthrough Device designation, this program has demonstrated its success and broad impact on regulatory pathways and intended uses.
By actively engaging with industry stakeholders, the FDA remains committed to advancing public health and ensuring the availability of cutting-edge medical technologies. In this article, we will explore the eligibility criteria, benefits, expedited development process, enhanced collaboration with the FDA, market differentiation, case study examples, challenges, and the impact of the Breakthrough Device Designation on health equity and addressing health disparities. This informative piece will shed light on the significance of this program in fostering medical innovation while maintaining the highest standards of patient care.
Background and Purpose of the Breakthrough Devices Program
The FDA's Breakthrough Devices Program serves as a pivotal initiative to fast-track the development and review of innovative medical devices that promise enhanced treatment or diagnosis for life-threatening or irreversibly debilitating diseases. This program supports devices that have the potential to provide significant advantages over existing alternatives, or that offer new solutions for patients with no other treatment options. An example of the program's impact is the development of an implantable device that delivers naloxone during an opioid overdose. The urgency of addressing opioid-related emergencies is underscored by the rise of potent synthetic opioids in the U.S., which can lead to rapid and unpredictable overdoses, often occurring when individuals are alone and unable to seek help. Another project that underscores the program's importance is a device addressing dysmenorrhea, a condition affecting countless individuals globally, with limited treatment options. This device aims to alleviate severe menstrual pain that, in some cases, only opioids can mitigate. As of June 30, 2023, the FDA's Center for Devices and Radiological Health (CDRH) has granted Breakthrough Device designation to 831 devices, with 77 of those having received marketing authorization. These figures not only demonstrate the program's success but also its broad spectrum, influencing a wide range of regulatory pathways and intended uses. By actively engaging with industry stakeholders, the FDA remains committed to advancing public health, ensuring that medical devices on the market are safe and effective while fostering the availability of cutting-edge medical technologies.
Eligibility Criteria for Breakthrough Device Designation
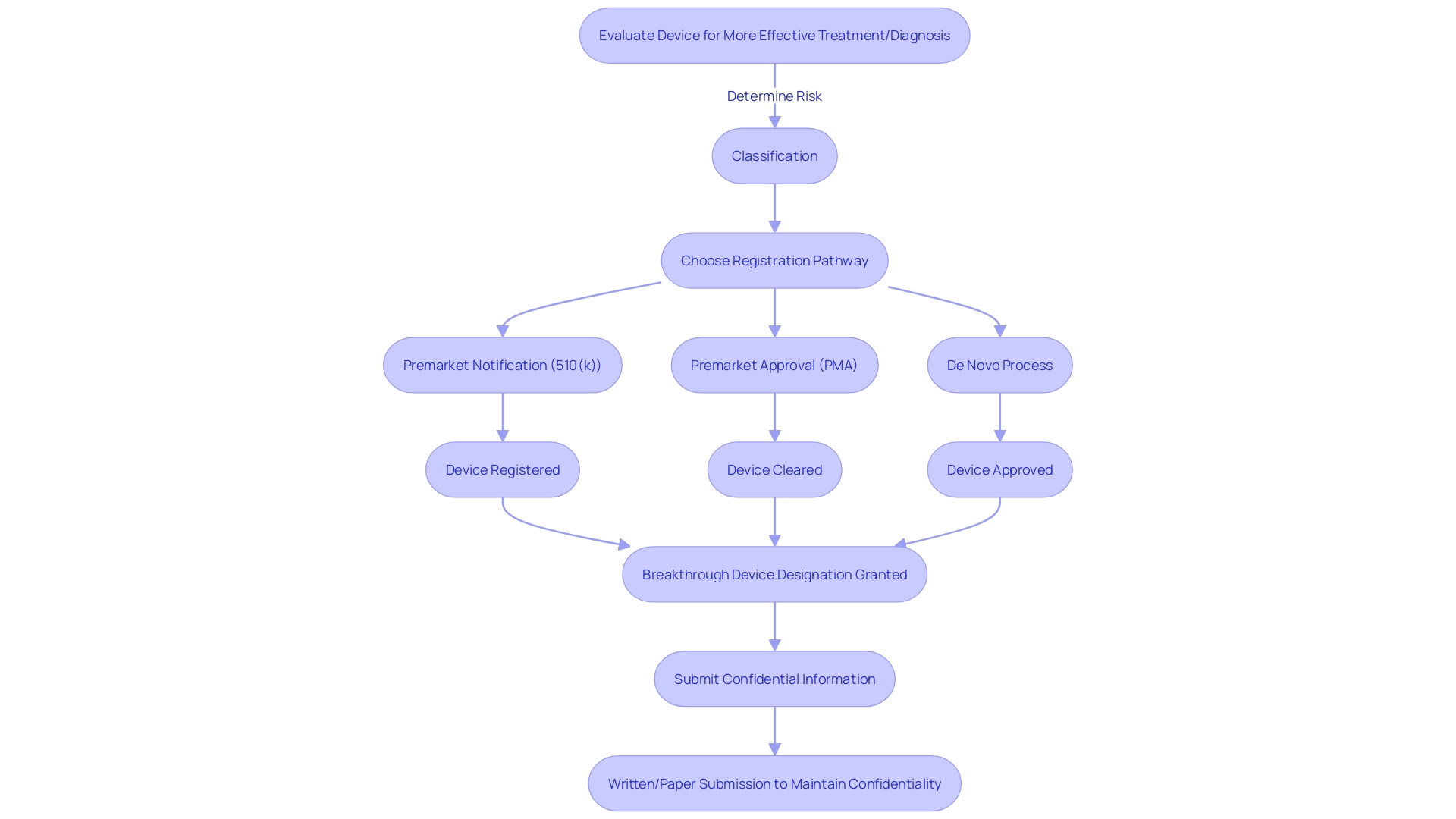
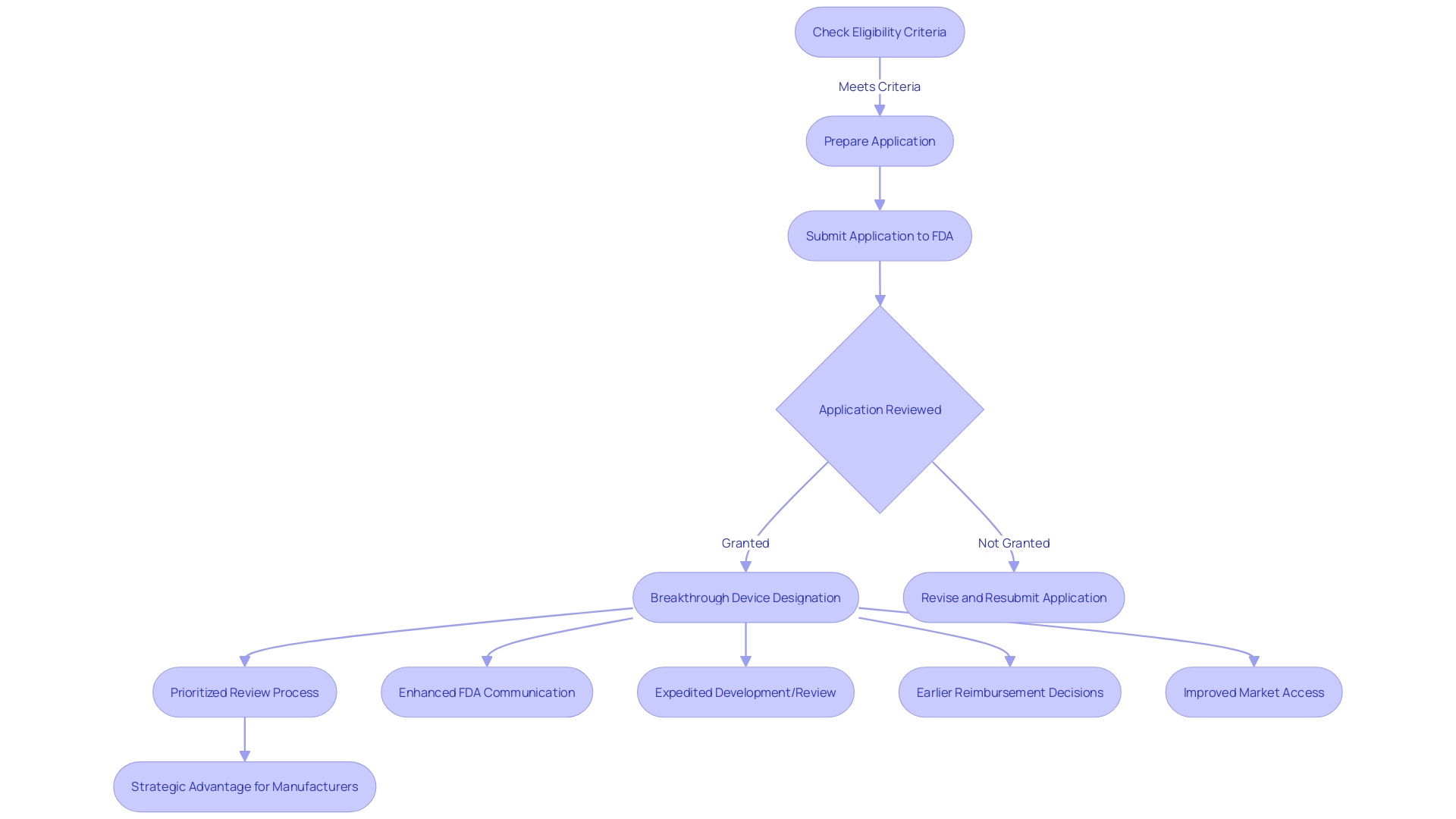
To achieve FDA Breakthrough Device Designation, medical devices must adhere to strict eligibility criteria. Devices must demonstrate the potential for more effective treatment or diagnosis than currently available options. This involves a rigorous evaluation process to determine the device's classification based on patient risk, followed by choosing the appropriate registration pathway—be it a Premarket Notification (510(k)), Premarket Approval (PMA), or the De Novo process. Regulatory professionals recognize these terms—Registered, Cleared, Approved, and Granted—as indicators of a device's status with the FDA. Understanding these distinctions is crucial for the marketing and legal use of medical devices in the United States. When submitting comments or information to the FDA regarding device designation, confidentiality must be maintained; any sensitive information should be submitted as a written/paper submission to ensure it does not become public.
Benefits of Breakthrough Device Designation
Securing the FDA Breakthrough Device Designation is a significant milestone for medical device manufacturers, aiming to expedite the development and review processes. It is imperative to understand that medical devices are categorized into three classifications by the FDA, each reflecting a varying degree of patient risk. Determining the correct classification is the first step before selecting an appropriate registration pathway, which could be Premarket Notification (510(k)), Pre-Market Approval (PMA), or the De Novo process. Once a device is FDA Cleared, Approved, or Granted through the De Novo process, it can be legally marketed in the U.S.
The advantages of receiving the Breakthrough Device Designation are manifold. It allows manufacturers to engage in more effective communication with the FDA, facilitating a collaborative approach to the development process. This can lead to a swifter availability of the device to patients, potentially improving patient outcomes and addressing unmet medical needs. Moreover, this designation can streamline the reimbursement process, a critical factor in the successful adoption and widespread use of new medical technologies.
Understanding these pathways and terminologies is crucial for regulatory professionals who navigate the intricate landscape of medical device approval. Additionally, it's important to note that public comments and submissions to the FDA are made available to the public, underscoring the necessity for discretion when sharing confidential information. This transparency in the regulatory process is part of the FDA's commitment to safeguarding public health while also promoting innovation in the field of medical devices.
Expedited Development and Review Process
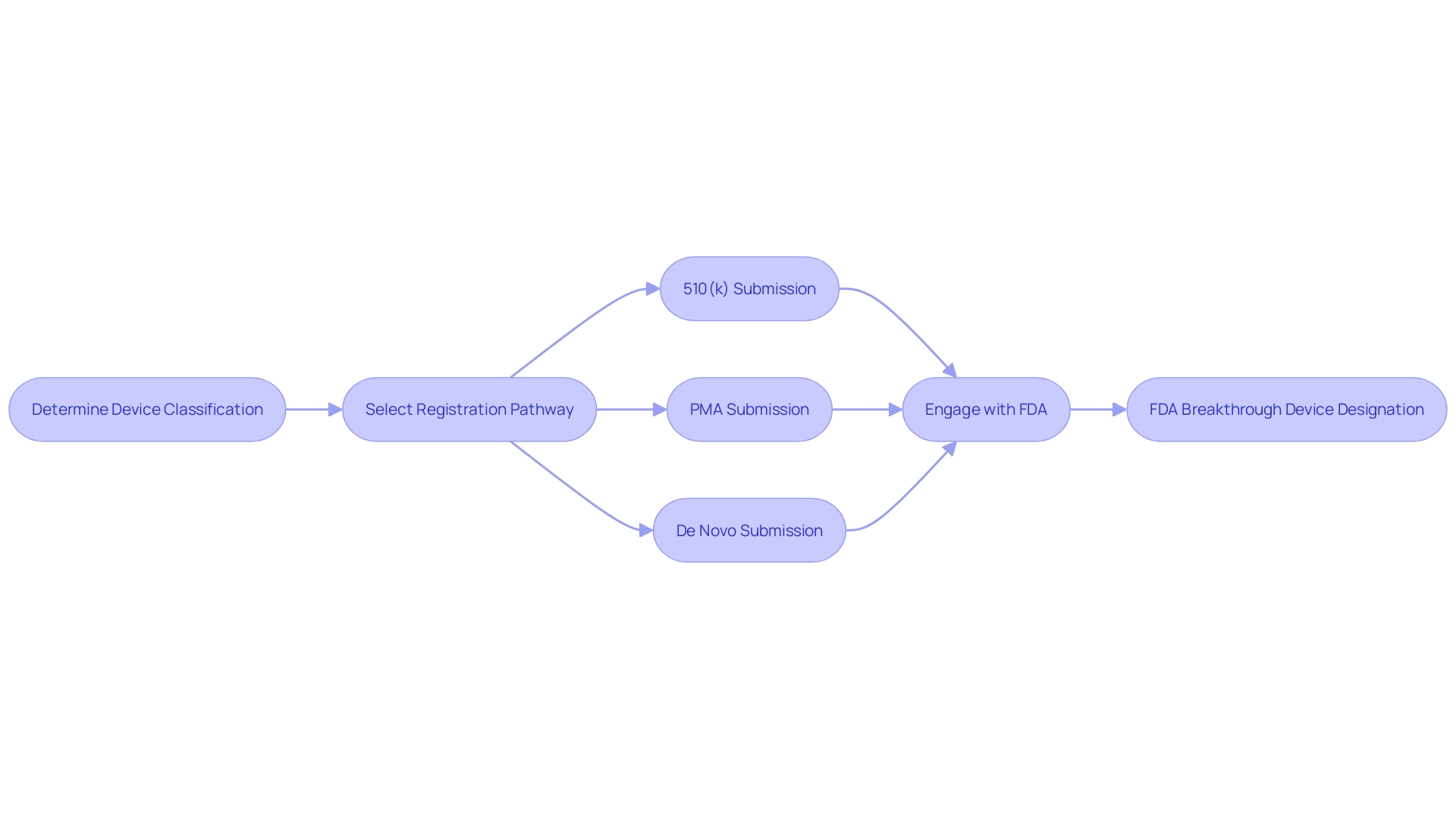
The FDA Breakthrough Device Designation represents a transformative approach to expediting the development and review of medical devices that hold the promise of more effective treatment or diagnosis of life-threatening or irreversibly debilitating diseases. It is crucial to comprehend the significant distinctions in the FDA's device classification system, which categorizes devices based on their associated patient risk into three levels. Once classified, developers choose the appropriate regulatory pathway: Premarket Notification (510(k)), Premarket Approval (PMA), or De Novo process. To legally market a device in the United States, it must be FDA Cleared, Approved, or in the case of a De Novo process, Granted. The nuances between these terms—Registered, Cleared, Approved, and Granted—are often a source of confusion but are essential for regulatory professionals to understand and accurately navigate.
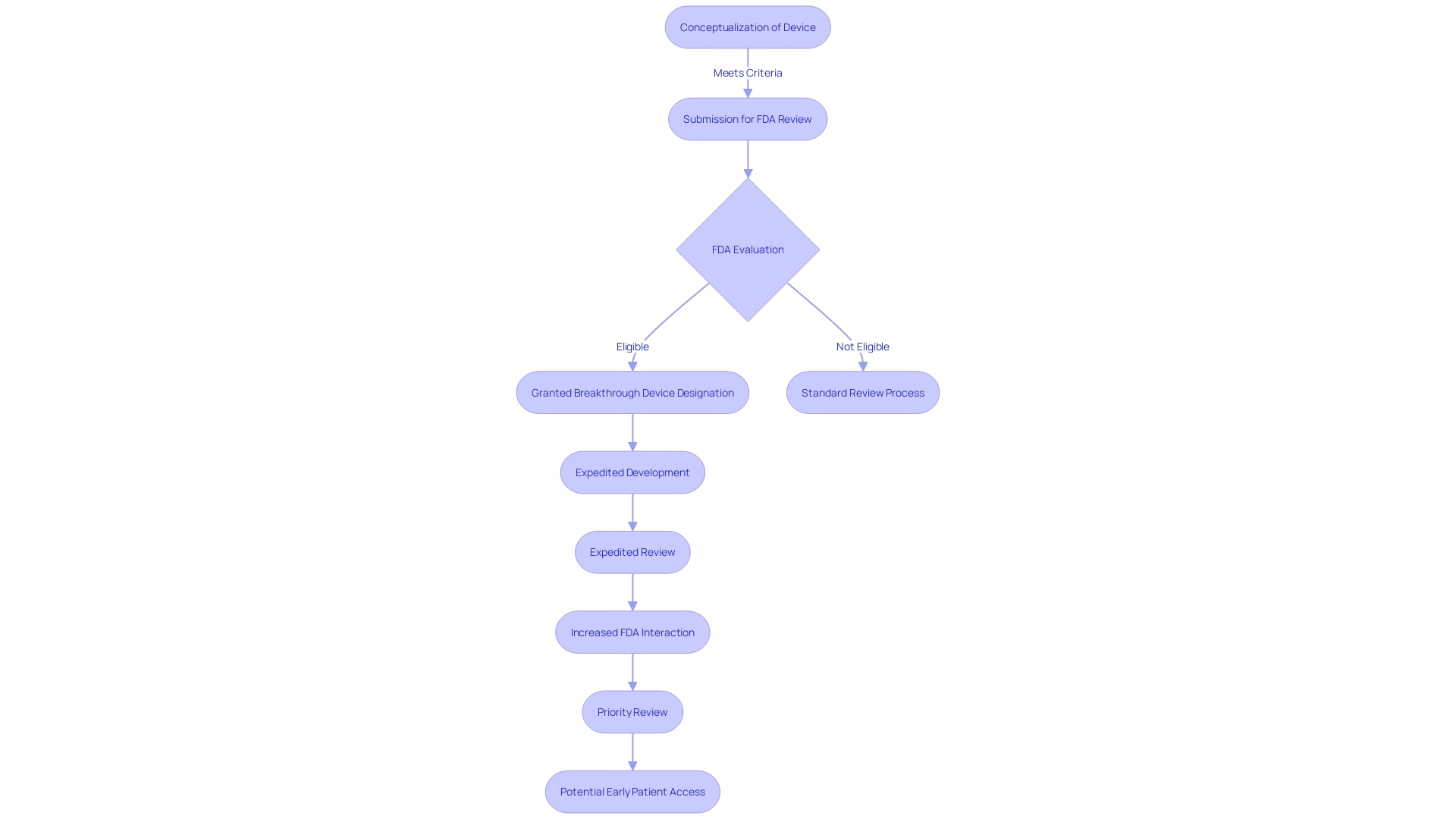
The expedited process afforded by the Breakthrough Device Designation ensures that innovative medical devices reach patients swiftly without compromising the FDA's high standards for safety, effectiveness, and security. This streamlined pathway is not only a boon for patients awaiting new therapies but also for medical device developers striving to fulfill urgent healthcare needs. In an era where medical advancements occur at a rapid pace, such designations are pivotal in fostering the development of groundbreaking technologies while maintaining the integrity and safety of the healthcare system.
Enhanced Collaboration and Communication with FDA
The FDA Breakthrough Device Designation is a pivotal catalyst for expediting the development and review of innovative medical devices that hold the potential to provide more effective treatment or diagnosis for life-threatening or irreversibly debilitating diseases. This program enhances the dialogue between device manufacturers and the FDA, offering the opportunity for companies to engage with the agency early and often throughout the device development process. By leveraging this interactive communication, manufacturers can better navigate the FDA's stringent requirements, aligning their submissions more closely with the agency's expectations for safety and efficacy.
To successfully market a medical device in the U.S., manufacturers must understand the FDA's classification system, which assigns devices to one of three categories based on patient risk. Determining the appropriate classification is the first step before choosing a regulatory pathway, such as a Premarket Notification (510(k)), Premarket Approval (PMA), or the De Novo process. The coveted Breakthrough Designation can facilitate a swifter path to market by helping manufacturers to clarify and streamline the data needed not just for FDA approval, but also to satisfy the coverage and reimbursement criteria set by payors like CMS and private health plans.
Despite the rigor of the FDA's approval process, there can be a disconnect between the data required for FDA clearance and the data needed by payors for coverage decisions. This gap can lead to delays or even denials in device coverage, impacting patient access. Therefore, the Breakthrough Device Designation becomes critically important as it helps bridge this gap, ensuring that the data submitted satisfies the broad spectrum of stakeholders involved in bringing a medical device to the patients who need it most.
The FDA's commitment to protecting public health through stringent regulation of medical devices ensures the safety, effectiveness, and security of these products. The Breakthrough Device Designation underscores the agency's dedication to fostering medical innovation while maintaining the highest standards of patient care.
Flexible Clinical Trial Design and Early Patient Access
The FDA's Breakthrough Device Designation is a pivotal program that fosters the advancement of novel medical technologies. It offers a pathway for expedited development and review of devices that have the potential to significantly enhance patient care. A key advantage of this designation is its support for adaptive trial designs, which are essential for swiftly gathering clinical evidence. These designs are not rigid; they allow for modifications to the trial procedures based on interim results, which helps in identifying the efficacy of a device sooner, reducing the time to market.
Furthermore, the Breakthrough Device Designation facilitates earlier access for patients to innovative treatments. As quoted by industry experts, partnerships with the FDA are crucial and productive, allowing companies to streamline interactions and efficiently introduce breakthrough products to the market. The importance of this can be seen in the context of emerging medical devices, such as leadless pacemakers, which eliminate some of the complications associated with traditional devices.
In light of the fact that the FDA's primary role is to ensure the safety and efficacy of medical devices, the designation also implies a level of confidence in the device's potential. This confidence may translate into swifter adoption by payors and healthcare providers after FDA approval, potentially reducing delays in patient access to new treatments. However, manufacturers must be mindful that the data submitted to the FDA might differ from what payors require for coverage decisions, underscoring the importance of comprehensive data collection and analysis.
Industry events, such as the MedTech Regulatory Intelligence Summit, emphasize the dynamic regulatory landscape and the FDA's commitment to collaborating with professionals to address challenges in bringing innovative medical devices to those in need. The Breakthrough Device Designation, with its intent to provide more effective treatments or diagnoses for life-threatening or irreversibly debilitating diseases, represents a significant stride toward enhancing patient quality of life and establishing long-term clinical efficiencies.
Market Differentiation and Potential for Earlier Reimbursement
The FDA Breakthrough Device Designation stands as a pivotal achievement for medical device manufacturers, ushering in not only a competitive edge but also a smoother path toward reimbursement. This esteemed designation is reserved for devices that promise more effective treatment or diagnosis of life-threatening or irreversibly debilitating diseases. For manufacturers, it translates into prioritized review processes and enhanced communication with the FDA, facilitating expedited development and review of their groundbreaking technologies.
The designation's influence extends beyond regulatory interactions. It signals to healthcare payers a device's transformative potential, which can lead to earlier reimbursement decisions, enhancing the financial viability and market uptake of these innovations. By distinguishing devices with this designation, manufacturers are better positioned to navigate the complex landscape of medical device classifications and regulatory pathways—a landscape where proper classification and pathway selection, whether it be Premarket Notification (510(k)), Pre-Market Approval (PMA), or De Novo process, is crucial for market access. This strategic advantage is underscored by the FDA's commitment to ensuring the safety, effectiveness, and security of medical devices, which aligns with the goals of providers and patients alike to adopt novel and beneficial medical technologies.
Ultimately, the Breakthrough Device Designation stands as a testament to a device's potential to significantly impact clinical practice and patient care, fostering an environment where innovation thrives and is recognized for its ability to address the most challenging medical needs.
Case Study Examples: Success Stories of Breakthrough Devices
The FDA's Breakthrough Device Designation represents a pivotal shift in how innovative medical devices can expedite their journey from conceptualization to clinical application. This designation is granted to devices that have the potential to significantly improve treatment or diagnosis of life-threatening or irreversibly debilitating conditions and for which no adequate alternatives exist. To qualify, a device must demonstrate substantial benefits over existing approved options, potentially reducing the need for hospitalization, enhancing quality of life, or offering long-term clinical efficiencies. This initiative not only accelerates the development and review process but also provides manufacturers with the FDA's guidance earlier in the process, paving the way for quicker patient access.
One notable example is a device designed to alleviate symptoms of left ventricular failure, which underscores the transformative impact of the designation. By supporting the heart's pumping function, the device significantly enhances patient quality of life. Such advancements are part of a broader movement, fueled by rapid technological progress and growing demand for tech-enabled healthcare solutions. Events like the MedTech Regulatory Intelligence Summit further this progression by fostering dialogue between the FDA and industry experts on best regulatory practices.
In addition to facilitating innovation, the FDA ensures public health by overseeing the safety and effectiveness of a wide range of products, from drugs and medical devices to food and cosmetics. The public can engage with the agency's regulatory processes through open comment periods, which are crucial for maintaining transparency and accountability. However, participants are advised to withhold confidential information from their submissions to protect their privacy.
The Breakthrough Device Designation is a testament to the FDA's commitment to fostering medical innovation while maintaining rigorous safety standards. It serves as a beacon for healthcare systems, advocacy groups, and manufacturers, aligning regulatory pathways with the rapid pace of technological advancement to meet the urgent needs of patients.
Challenges and Considerations for Maintaining Breakthrough Designation
The FDA Breakthrough Device Designation brings with it a pathway to expedite the development and review process for innovative medical devices. However, manufacturers must meticulously adhere to specific post-approval requirements, including vigilant monitoring of device performance and timely reporting of any adverse events. This close scrutiny is designed to mitigate potential risks inherent in new technologies and to maintain the integrity and safety of breakthrough devices once they reach the market. Compliance with these post-market surveillance measures is critical, as any lapses could compromise patient safety and the device's designation status. Navigating the post-approval landscape requires manufacturers to be proactive and transparent, particularly in the management of any confidential information that may be disclosed in public documents or communications with the FDA. As such, manufacturers must balance the pursuit of innovation with a commitment to regulatory vigilance and patient safety.
Impact on Health Equity and Addressing Health Disparities
The FDA Breakthrough Device Designation is an instrumental policy in addressing health equity and mitigating disparities within healthcare. By fostering the development and expedited review of innovative medical devices, this designation stands as a beacon of progress for underserved populations. Central to its ethos is ensuring that clinical studies encompass a broad spectrum of the intended user base, particularly focusing on disease burden, physiological considerations, and the suitability of technology for diverse groups. This inclusive approach to device approval is poised to diminish gaps in healthcare outcomes and promote equitable treatment.
Recent FDA initiatives, such as the 'Home as a Healthcare Hub,' underscore the commitment to integrating the home environment into healthcare delivery, thereby increasing accessibility for all, especially for racial and ethnic minorities, rural residents, and lower-income groups. These efforts aim to reimagine healthcare by addressing the systemic challenges and bridging the divide that has historically placed certain populations at a disadvantage. The FDA solicits public input to shape these strategies further, recognizing the importance of community feedback in creating a more inclusive healthcare system.
Public comments are essential in this dialogue, providing the FDA with diverse perspectives that can guide the evolution of policies geared towards health equity. Stakeholders are encouraged to participate in this discourse, with the understanding that their contributions will be part of the public record, aimed at fostering an environment where innovative medical devices are within reach for every segment of the population.
As part of its strategic priorities, the FDA's focus on health equity is not just a commitment on paper but a call to action for all involved in healthcare - from policymakers to device manufacturers and the public at large. The agency's approach is a testament to the potential of the Breakthrough Device Designation to catalyze change and ensure that advancements in medical technology translate into tangible benefits for those who need them most.
Conclusion
The FDA's Breakthrough Devices Program expedites the development and review of innovative medical devices for life-threatening or irreversibly debilitating diseases. It supports devices with new solutions or significant advantages over existing alternatives. This designation allows manufacturers to collaborate effectively with the FDA, accelerating device availability and improving patient outcomes.
The streamlined process maintains high standards of safety and effectiveness.
The Breakthrough Device Designation enhances communication between manufacturers and the FDA, facilitating early engagement throughout the development process. It provides a competitive edge and smoother reimbursement path for devices that promise more effective treatment or diagnosis of serious conditions. This program plays a vital role in fostering innovation while ensuring regulatory compliance.
In conclusion, the FDA's Breakthrough Device Designation program is instrumental in expediting the development and review of innovative medical devices. It promotes collaboration, accelerates availability, and improves patient outcomes. This designation supports medical innovation while upholding rigorous safety standards, benefiting both manufacturers and patients.




