Introduction
The FDA's Breakthrough Devices Program is a critical initiative aimed at accelerating the development and review process for innovative medical devices. Designed to offer more effective treatment or diagnosis for life-threatening or irreversibly debilitating diseases, these devices undergo a rigorous evaluation to qualify for the Breakthrough Device designation. By reducing the time it takes for these crucial medical devices to become available to patients, the program addresses the scarcity of tailored devices for certain populations, such as children.
Furthermore, the program not only aids in speeding up the regulatory approval process but also potentially aligns the interests of different stakeholders, including manufacturers, payors, and healthcare providers. In this rapidly evolving digital health space, the Breakthrough Devices Program plays a vital role in facilitating the swift transition of medical devices from conception to clinical use. Join the upcoming webinar on November 14, 2023, to gain further insights into the updated final guidance for the Breakthrough Devices Program and deepen your understanding of its impact on the healthcare innovation landscape.
Background and Purpose of the Breakthrough Devices Program
The FDA's Program for Advancing Innovative Medical Technology is a crucial initiative intended to expedite the development and review process for cutting-edge medical devices. These instruments have the potential to offer more efficient treatment or diagnosis for life-threatening or irreversibly debilitating human diseases or conditions. To be eligible for the Device Designation, a product must meet specific criteria, such as providing significant advantages over existing alternatives or representing the best interests of patients. The program's objective is to decrease the time it takes for these vital healthcare tools to become accessible to patients who require them most.
The significance of the Program for Advancement Gadgets is emphasized by the reality that specific groups, like children, frequently encounter a shortage of healthcare tools customized to their distinct requirements. This scarcity is because, in some measure, of both a scarcity of technical advancement and a perceived low return on investment for pediatric apparatus, which enhances the value of the accelerated pathway provided by the Program for Advancement of Innovative Devices.
Furthermore, the FDA's responsibility goes beyond the endorsement of healthcare instruments. It encompasses the assurance of the safety, effectiveness, and security of these products. The agency also plays a vital part in public health by regulating food, cosmetics, and other products, thus ensuring the overall well-being of the population.
In recent times, the digital health space has seen rapid innovation, partly propelled by the COVID-19 pandemic. This has led to a greater focus on tech-enabled and virtual healthcare solutions. It is in this framework that the Program for Advancement Tools operates, facilitating the rapid transition of healthcare instruments from conception to clinical use.
For medical device manufacturers, navigating the regulatory landscape can be complex, as the data required for FDA approval may differ from the data needed by payors to make coverage decisions. This discrepancy can result in delays or denials in coverage, despite FDA approval. Hence, the Devices Program not only helps in expediting the regulatory approval process but also potentially supports in aligning the interests of different stakeholders, including manufacturers, payors, and healthcare providers.
The upcoming webinar on November 14, 2023, is set to offer further insights into the updated final guidance for the Devices Program, providing an invaluable opportunity for stakeholders to deepen their understanding of the program and its impact on the healthcare innovation landscape.
Eligibility Criteria for Breakthrough Device Designation
In order to meet the strict criteria, medical equipment must be eligible for the FDA Breakthrough Designation. These eligibility requirements are focused on the equipment's potential influence on healthcare and patient outcomes. Specifically, the apparatus must aim at a life-threatening or irreversibly debilitating condition, for which there are no existing approved or cleared treatment alternatives. In addition, the equipment should provide significant benefits over current alternatives, such as the ability to decrease or eliminate hospitalization, improve patient quality of life, allow self-management of care, or create long-term clinical efficiencies. The designation is granted in the interest of patients, signifying that the equipment represents a substantial improvement over current treatment options.
Benefits of the Breakthrough Devices Program for Medical Innovations
The FDA's Breakthrough Devices Program plays a crucial role in promoting innovations in the healthcare sector, especially in the advancement and implementation of life-saving technologies. Among the benefits it provides to the healthcare equipment sector is the chance for priority assessment, which simplifies the evaluation procedure, enabling potentially game-changing technologies to reach patients more expeditiously. Improved communication channels between the FDA and developers facilitate a more collaborative approach, fostering dialogue and understanding that can accelerate the path to market. Moreover, early market access is another significant benefit, as it can lead to quicker adoption and integration into healthcare systems, ultimately benefiting patients with critical medical needs.
Highlighting the significant influence of this program, take into account the scenario of an implantable apparatus intended to identify and react to opioid overdoses with the administration of naloxone, a life-saving intervention that can restore normal breathing. The urgency for such innovations is underscored by the rapid onset of opioid overdoses, often occurring when individuals are alone. The Devices Program could speed up the availability of such crucial solutions.
Another instance is the creation of individualized deep brain stimulation apparatus for patients with Parkinson's disease. Tailored electrical stimulation can greatly reduce the time sufferers experience debilitating symptoms. This approach illustrates the kind of innovative healthcare solutions that can emerge under the auspices of the Breakthrough Devices Program, highlighting its potential to transform patient care.
To comprehend the extent of innovation in the domain, contemplate the swift progressions in healthcare technology, driven by the amalgamation of software and hardware in devices. Companies adept in this integration possess a competitive advantage, as evidenced by industry leaders who offer groundbreaking systems and solutions. ISO 13485 certification, indicative of a commitment to quality management, is often a benchmark for these development companies, which maintain a track record of successful product launches and demonstrate the capability to navigate the complex journey from concept to market.
In summary, the Devices Program not only accelerates the approval process but also fosters a symbiotic relationship between innovators and regulators. This connection is vital in advancing technologies that have the potential to make significant impacts and improve patient outcomes across a variety of conditions.
Recent Updates to the Breakthrough Devices Program Guidance
To keep up with the rapid advancements in the field of healthcare technology, the U.S. Food and Drug Administration (FDA) regularly updates its guidance for the Program focusing on innovative devices. These modifications are vital for companies aiming for Breakthrough Device Designation as they can significantly impact the trajectory of healthcare innovation. The most recent revisions encompass adjustments to the eligibility criteria, the review process, and post-market obligations, ensuring that the program remains effective within the dynamic realm of healthcare equipment development. Medical companies must be vigilant and adapt to these changes to facilitate expedited development and deployment of groundbreaking technologies that can enhance patient care and treatment outcomes. The FDA's dedication to public health is apparent in its thorough supervision of the safety, effectiveness, and security of healthcare instruments, thus promoting an atmosphere where innovative solutions can flourish while maintaining rigorous safety standards.
Impact on Health Equity and Addressing Health Disparities
The FDA's Breakthrough Devices Program is crucial in promoting the accessibility of innovative technologies for the health of underserved populations, thus contributing to the reduction of health disparities. For instance, recent initiatives have focused on comprehending the unique healthcare needs and barriers faced by diverse groups. A pilot study involving Native Hawaiian, Pacific Islander, Filipino, and white patients hospitalized with diabetes revealed a substantial interest in clinical trials, highlighting the need for support and trust-building measures to facilitate participation.
In a bid to advance health equity, the FDA is actively seeking feedback to ensure that clinical studies reflect the intended use population, considering disease burden, physiology, and technology. This approach is aligned with the FDA's strategic priority to enhance health equity from 2022 to 2025. The FDA emphasizes the importance of generating clinical data that are generalizable and representative of the population for which an instrument is intended.
Furthermore, the 'Home as a Health Care Hub' initiative is a testament to the FDA's commitment to reimagining home environments as a crux of the healthcare system. Imagining houses equipped with health tools that are smoothly integrated and user-friendly has the potential to transform healthcare provision, particularly for individuals with restricted entry to conventional healthcare settings.
Amid these efforts, diabetes stands out as a critical area where digital health technologies (DHTs) can significantly impact. With one in ten Americans affected by diabetes, and a significant portion unaware of their condition, there's a pressing opportunity for DHTs to facilitate early diagnosis and management, especially in socioeconomically disadvantaged or geographically isolated communities. The integration of DHTs in homes could greatly extend healthcare's reach, empowering individuals to take an active role in managing their health.
These case studies and initiatives highlight the FDA's commitment to guaranteeing that groundbreaking medical technologies not only encourage innovation but also tackle the crucial matter of health equity, ultimately enhancing healthcare outcomes for all segments of the population.
Case Studies: Successful Breakthrough Device Designations
The FDA Designation for Advancement provides a pathway to accelerate the development and review of tools that offer improved treatment or diagnosis for life-threatening or irreversibly debilitating diseases. To be eligible, it must distinguish itself from already approved alternatives by possibly reducing hospital stays, improving patient quality of life, facilitating self-care, or establishing long-term efficiencies.
Illustrating the profound impact of this program, consider the advancements in digital health, driven by rapid technological growth, increased data capabilities, and heightened demand for virtual healthcare solutions. These elements are converging to fuel innovation, as seen with mental health apps that integrate risk assessment, safety, and functionality to aid healthcare systems and consumers.
A prominent case is the National Institute of Mental Health's focus on implementing evidence-based treatments into practice. The organization emphasizes the translation of scientific breakthroughs into policies that can improve the quality of care for mental illness, a condition affecting over 20% of U.S. adults.
Furthermore, the FDA's dedication to health equity is apparent in its preliminary advice to incorporate healthcare instruments that can be advantageous to populations experiencing health inequalities within the Innovative Devices Program. This initiative underscores the FDA's role in safeguarding public health by ensuring the safety and effectiveness of health innovations.
To stay abreast of these developments, the MedTech Regulatory Intelligence Summit serves as a nexus for regulatory professionals and FDA members to discuss best practices and challenges in this dynamic sector. The upcoming FDA webinar on updated final guidance will further clarify the criteria for the innovative technology designation, reinforcing the agency's support for transformative healthcare equipment that prioritize patient well-being.
Process for Requesting and Receiving Breakthrough Device Designation
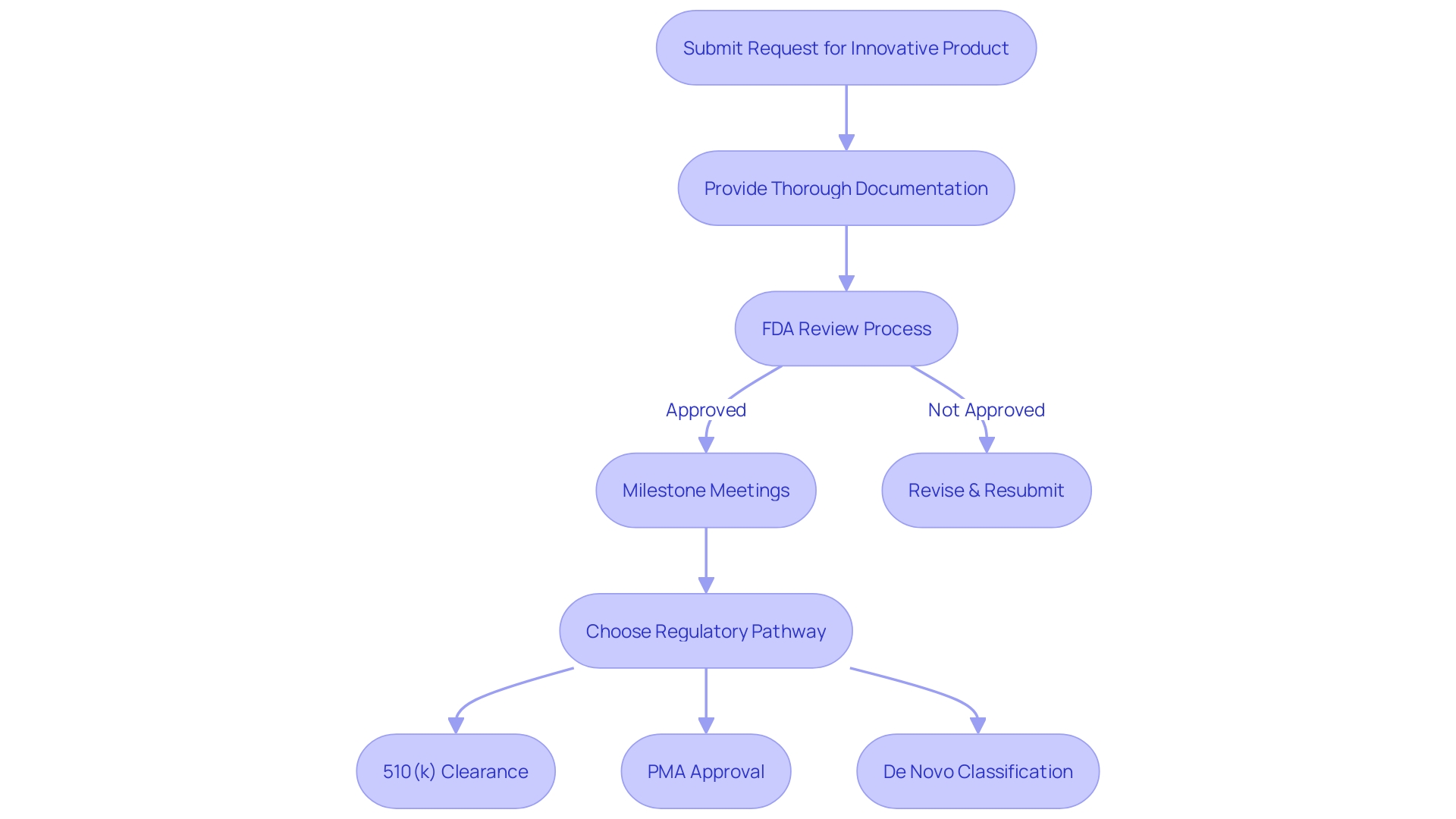
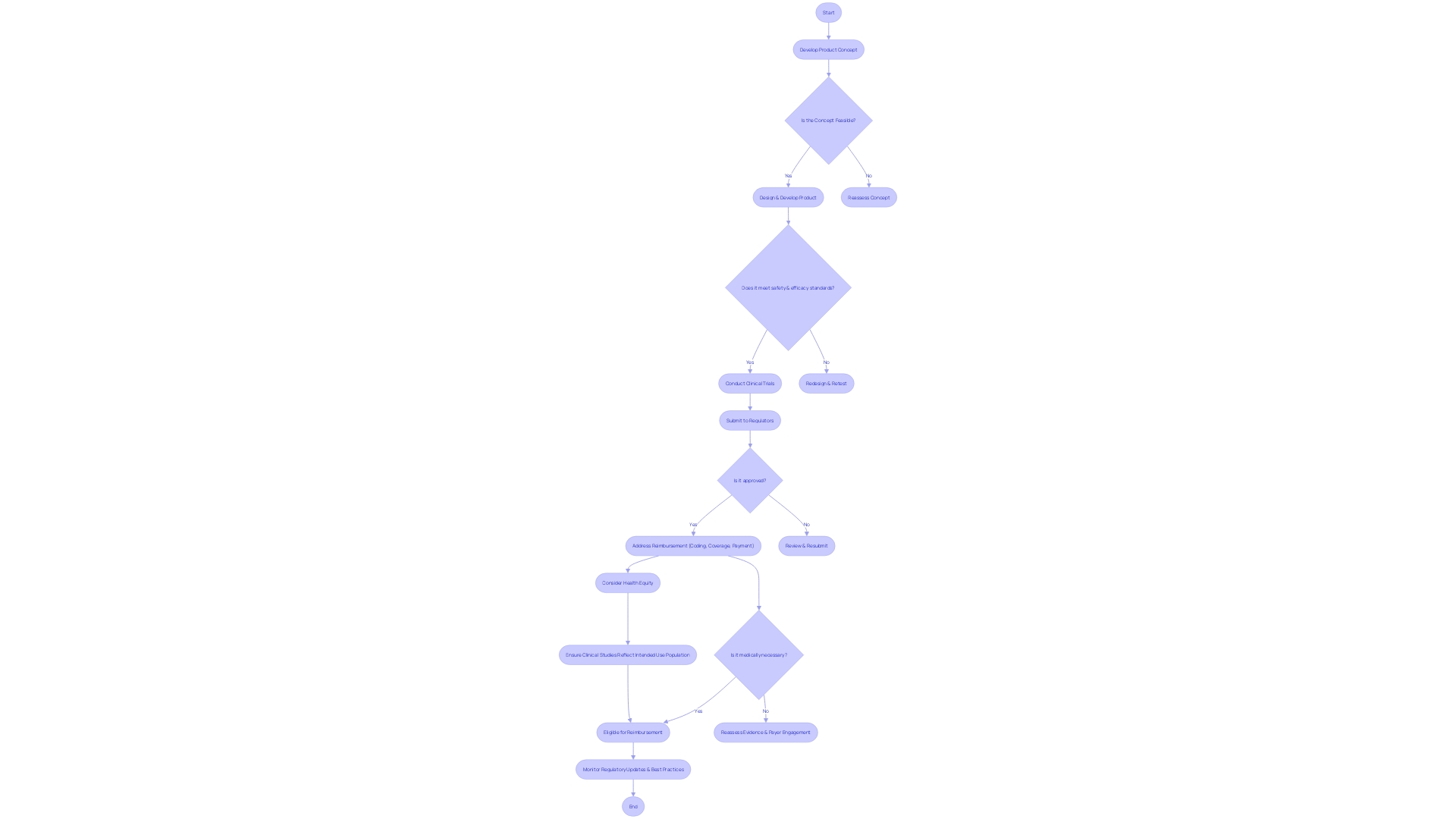
To accelerate the advancement and evaluation of tools that can greatly enhance the well-being of individuals with severe or irreversible debilitating ailments, the FDA has implemented the Innovative Device Designation. This differentiation is vital for medical instruments that have the potential to offer more efficient treatment or diagnosis, with no authorized or cleared alternatives, or that can provide significant benefits over existing options. In order to obtain this designation, manufacturers must submit a thorough Request for an Innovative Product that encompasses a categorization of the item, an elaborate explanation of the product and its constituents, progress reports, manufacturing procedures, and methods of operation. The request must also describe the ailment or state the apparatus is meant to tackle, alongside the patient population it serves.
The FDA's review process for these requests is meticulous, assessing each submission's potential to provide significant benefits to patients. Upon approval, the designation can lead to more efficient development and evaluation processes. Manufacturers can leverage a platform technology designation for efficiencies in drug development, manufacturing, and review processes. The FDA also encourages discussions during milestone meetings to optimize the submission and review of the Breakthrough Device Request, with clear timelines outlined for review. Additionally, the FDA offers webinars and educational resources to provide further guidance on the updated final guidance and the nuances of the designation process.
It is essential for companies to understand the FDA's classification system and choose the appropriate regulatory pathway—be it Premarket Notification (510(k)), Pre-Market Approval (PMA), or the De Novo process. The terminologyâRegistered, Cleared, Approved, and Grantedâreflects the different levels of FDA assessment and authorization required before an item can be marketed in the United States. This procedure guarantees that the groundbreaking instruments fulfill the demanding criteria of safety and efficiency established by the FDA, ultimately protecting public health while promoting advancement in healthcare technologies.
FDA Support and Resources for Breakthrough Devices
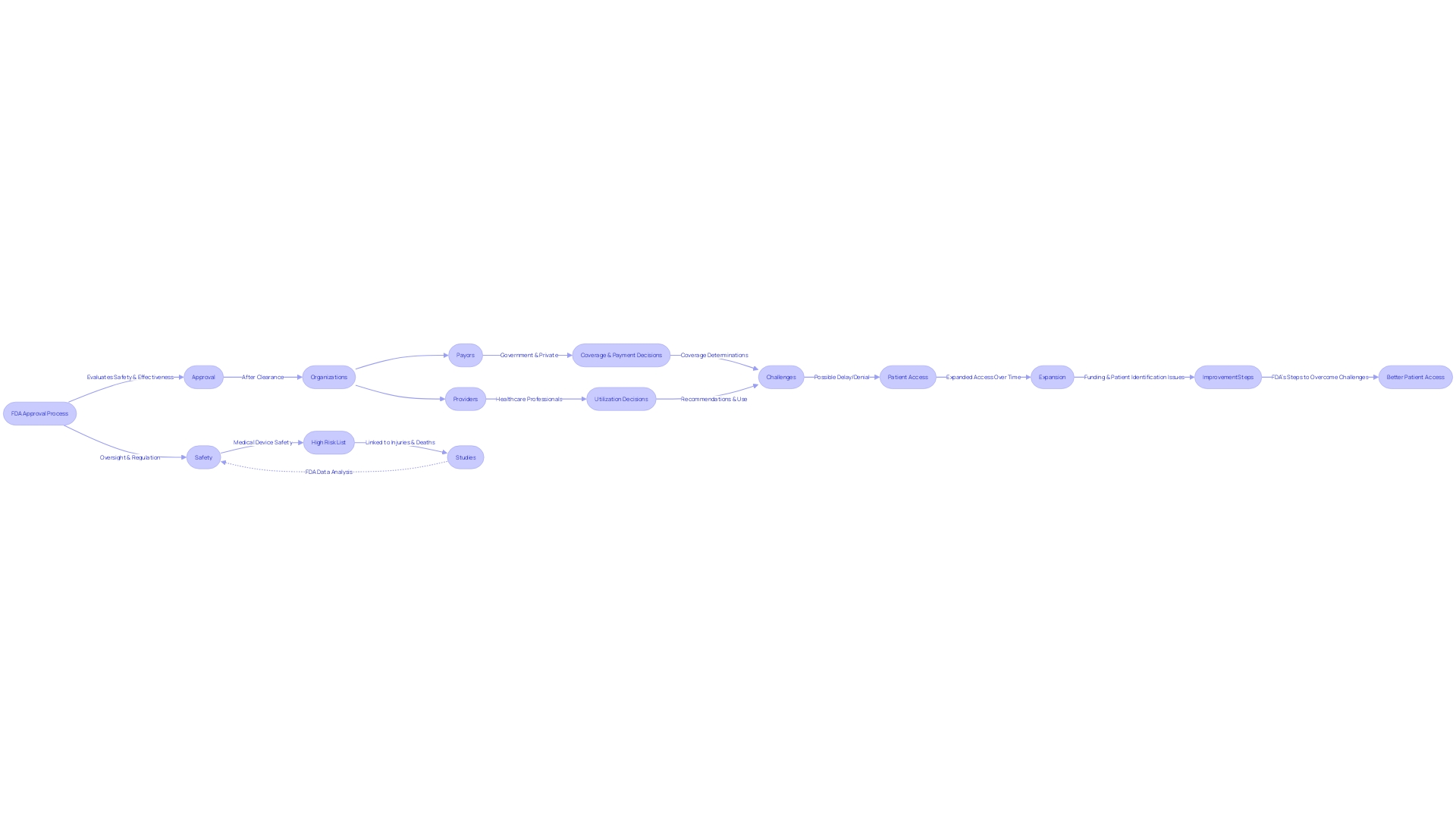
The FDA's Breakthrough Program accelerates the advancement and evaluation of innovative tools that offer improved care or diagnosis for life-threatening or irreversibly debilitating diseases. Under this program, manufacturers can benefit from proactive engagement with the FDA, including priority review and interactive communication regarding development and clinical trial protocols. This collaborative approach can lead to earlier access to high-quality, safe, and effective healthcare technology for patients, potentially transforming the landscape of treatment options.
However, even with FDA approval or clearance, the journey doesn't end there. Medical equipment frequently faces obstacles in coverage, payment, and utilization decisions by payors and healthcare providers. Payors, including CMS, private health plans, and health technology assessment groups, require data that may differ from what was submitted to the FDA, leading to possible delays or denials in coverage. This underscores the need for manufacturers to strategically navigate not only the regulatory landscape but also the complex reimbursement environment to ensure patient access to breakthrough technologies.
With the FDA's dedication to public health and safety, ensuring the effectiveness and security of healthcare equipment is crucial. The organization's regulatory oversight extends beyond healthcare equipment to include drugs, biological products, food supply, cosmetics, dietary supplements, and electronic radiation-emitting products. The FDA's actions are guided by a profound comprehension of the healthcare landscape, and its policies are continually evolving to accommodate the rapid advancements in healthcare technology.
Challenges and Considerations for Seeking Breakthrough Designation
The FDA's Program is crucial in promoting innovation in the field of medicine, particularly for tools that offer improved treatment or diagnosis for severe or permanently disabling ailments. Nevertheless, obtaining Device Designation necessitates navigating a intricate landscape of proof and data requirements. As medical tools progress, addressing health equity becomes crucial. The FDA's discussion paper highlights the necessity for clinical studies to mirror the intended use population, taking into account the disease burden, patient physiology, and the technology applied. These considerations ensure that outcomes are generalizable and representative of diverse patient groups, aligning with the FDA's strategic priority of advancing health equity. Moreover, the AHRQ's framework for assessing the safety and functionality of health apps exemplifies the broader trend of increased scrutiny on digital health innovations. The forthcoming MedTech Regulatory Intelligence Summit is an ideal forum for industry professionals to engage with the FDA and discuss best practices for navigating these regulatory challenges. The summit underscores the importance of keeping abreast of regulatory updates, such as the FDA's upcoming webinar on Breakthrough Device designation criteria. These criteria include significant advantages over existing alternatives and potential benefits such as reduced hospitalization and enhanced patient quality of life. The designation also takes into account objects with new intended uses or those employing a fundamentally different scientific technology. Such rigorous standards ensure that only the most promising and impactful devices gain expedited access to the market, ultimately benefiting patients' health and well-being.
Conclusion
In conclusion, the FDA's Breakthrough Devices Program accelerates the development and review process for innovative medical devices. It addresses the scarcity of tailored devices for specific populations and aligns the interests of stakeholders. The program facilitates the swift transition of devices from conception to clinical use in the rapidly evolving digital health space.
Recent updates to the program's guidance ensure its effectiveness in the dynamic realm of medical device development. These updates reflect the FDA's commitment to safeguarding public health and fostering an environment for cutting-edge solutions.
The program has a significant impact on health equity by addressing disparities and seeking feedback to ensure studies reflect the intended use population. It offers manufacturers proactive engagement with the FDA, leading to earlier access to safe and effective devices for patients.
While challenges exist in navigating evidence and data requirements, the Breakthrough Devices Program fosters medical innovations, addresses disparities, and ensures device safety and effectiveness.
Overall, the program provides a pathway to expedite the development of devices with significant clinical impacts, improving outcomes across medical conditions.




