Introduction
Clinical Research Organizations (CROs) play a crucial role in the realm of medical device clinical trials, offering specialized expertise and resources to navigate the complex journey from concept to market. These organizations ensure high standards of safety and efficacy while maneuvering through regulatory hurdles. With the ever-evolving landscape of healthcare innovation, CROs have become central actors in the dialogue between innovation, regulation, and patient care.
In this article, we delve into the significance of CROs, their role in the medical device industry's growth, their innovative methodologies in conducting clinical trials, and their contributions to advancing medical device research and enhancing patient outcomes. We also explore the challenges and opportunities in the CRO partnership landscape and the importance of ethical conduct and data integrity. Join us as we uncover the pivotal role of CROs in the dynamic world of medical device development.
Abstract
Clinical Research Organizations (CROss) are pivotal in the landscape of healthcare innovation, particularly in the arena of medical device clinical trials. CROss offer specialized expertise and resources to shepherd medical devices through the intricate journey from concept to market, navigating regulatory hurdles and ensuring high standards of safety and efficacy.
A testament to the complexity of this process can be seen in a Phase I oncology study involving 52 patients and an astounding 105 sites. Such expansive studies illuminate the challenges of resource allocation and underscore the dynamism of project management within clinical trials, where changes such as personnel turnover and site amendments can emerge as early as three months into a project.
The role of medical devices, as defined by the World Health Organization, spans a broad spectrum from simple implements like tongue depressors to sophisticated technologies like computer-assisted diagnostic tools. Each device, depending on its complexity and application, must pass through regulatory scrutiny before becoming an integral part of patient care, aiding in diagnosis, treatment, and improving quality of life.
Regulatory bodies such as the FDA in the United States categorize medical devices into three classes, reflecting their potential risks and necessitating varying degrees of control. While class one and two devices may proceed through streamlined processes like the 510(k) clearance, class three devices, which include life-sustaining implants, undergo more rigorous assessment due to their higher risk profile.
In recent years, there has been a concerted push for more efficient regulatory pathways, a movement hastened by the pressing demands of the COVID-19 pandemic. This drive towards streamlined processes aims to hasten the approval of medical devices, especially in burgeoning fields like digital health and personalized medicine, addressing urgent medical needs while maintaining regulatory compliance.
As innovations continue to evolve, so too does the regulatory landscape. Professors Dhruva, Kesselheim, and Redberg point out that the level of evidence underpinning FDA approvals can vary significantly, a factor of which medical professionals must remain cognizant. Meanwhile, the pharmaceutical industry contends with the balance between the costs of innovation and the imperative to provide affordable, accessible healthcare solutions.
Ultimately, CROs are not just facilitators of clinical trials; they are central actors in the ongoing dialogue between innovation, regulation, and patient care, striving to embed research into practice and systematically dismantle barriers to progress for the betterment of patients and the healthcare system at large.
Background
As the medical device sector advances, the role of Clinical Trial Companies (CTCs), particularly Contract Research Organizations (CROs), has become increasingly central. With over three decades of experience, organizations like CMIC Group underscore the value of CROss in the medical device industry's growth. They contribute to the entire pharmaceutical value chain, offering end-to-end solutions including development, manufacturing, and market entry strategies.
Such comprehensive services are vital for ensuring that medical devices meet the stringent standards of safety and efficacy before they reach patients.
Clinical trials are essential for determining the safety and effectiveness of medical devices, and the expertise of CROss is crucial in managing these complex processes. For instance, CMIC has been at the forefront in Japan, innovating and expanding its services to meet the evolving needs of medical device manufacturers, academia, and medical institutions. They tailor their solutions to address the specific challenges of bringing new medical technologies to market, enabling their clients to navigate the regulatory landscape and advance their products.
Moreover, the involvement of CTCs and CROs extends beyond development and testing. They play a pivotal role in addressing the logistical and ethical considerations of clinical trials, which can span across borders and involve diverse patient populations. Their expertise helps in bridging the gap between innovation and practical application, ensuring that medical breakthroughs are not only scientifically sound but also accessible and implementable in real-world settings.
In the context of the rapid changes within the healthcare field, where technology and digital solutions are becoming increasingly important, CTCs and CROss help in streamlining the process of bringing medical devices to market. Their capacity to integrate new technologies and adapt to the industry's dynamic nature positions them as indispensable partners in the development and implementation of medical advancements. Their contributions are not only instrumental in improving patient outcomes but also in shaping the future of healthcare delivery.
Methods
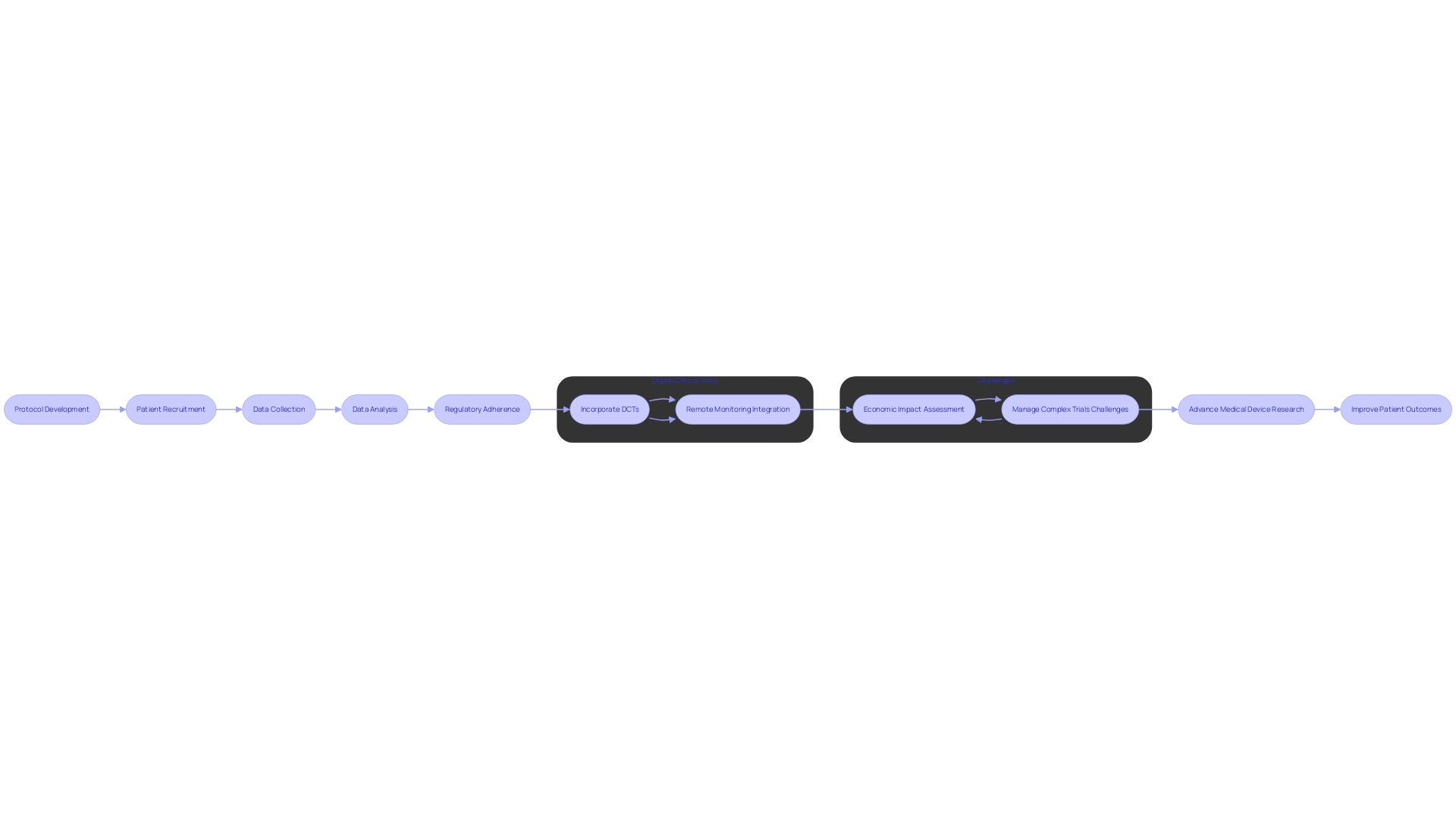
Clinical Research Organizations (CROs) have become pivotal in conducting medical device clinical trials, employing innovative processes and methodologies to ensure the efficiency and reliability of studies. Solutions Engineer Chris, with his extensive 13-year experience in the medical device industry, represents the expertise behind such organizations. CROss adeptly navigate through protocol development, meticulous patient recruitment, comprehensive data collection, and sophisticated analysis, all while strictly adhering to regulatory guidelines.
For instance, the utilization of Digital Clinical Trials (DCTs) has revolutionized patient inclusivity by allowing participants from remote locations to engage in trials without the need for physical site visits. This is exemplified by a Phase III cardiovascular trial where remote blood pressure monitoring devices were integrated, highlighting the cost-effectiveness and improved efficiency of such innovative approaches. Moreover, the Orchestras study showcases a CRO's capability to manage a hybrid clinical trial, including protocol finalization, IRB approvals, and holistic analysis, emphasizing the economic impact of new technologies on healthcare.
The advent of digital therapeutics, using software-based treatments over various devices, marks a significant shift in chronic disease management and neurological outcomes. Despite its potential to reduce patient costs, the novelty and limited insurance coverage pose challenges for widespread adoption and inclusion in clinical trials.
CROs are navigating these emerging complexities, as illustrated by the challenges faced during a multinational study involving a patient with a rare disease and the logistics of cross-border travel for participation. Their ability to manage multi-faceted trials demonstrates the critical role CROss play in advancing medical device research and enhancing patient outcomes.
Results
Clinical Research Organizations (CROs) have revolutionized the landscape of medical device clinical trials, markedly enhancing healthcare innovation. They offer medical device companies access to a wealth of specialized knowledge, experience, and the infrastructure required to effectively navigate the intricate regulatory environment of clinical trials. This partnership is instrumental in not only streamlining the trial process but also in optimizing the reliability of the results.
A pivotal aspect of CROs' contribution is their adeptness in patient recruitment strategies, which leads to the formation of diverse and broadly representative participant groups for studies. This is exemplified in the case of Decentralized Clinical Trials (DCTs), which leverage digital technologies like electronic patient-reported outcomes (ePROs), electronic clinical outcome assessments (eCOAs), and Internet of Things (IoT) integrated wearables to transcend traditional barriers of trial participation. For instance, Zelta's remote blood pressure monitoring for a Phase III trial exemplifies how virtual approaches can enhance data collection efficiency, expand patient reach beyond geographical constraints, and reduce trial costs.
In another example, the Orchestras study, managed by Curavit in collaboration with MedRhythms, underscores the importance of hybrid clinical trial models. These models blend on-site and remote trial activities to not only assess therapeutic efficacy but also analyze the economic impact on healthcare systems. Moreover, the use of digital therapeutics in clinical trials is garnering attention for its potential to manage chronic diseases and neurological conditions, promising improved patient outcomes and reduced healthcare costs.
As the industry evolves, the role of CROs in fostering innovative trial designs and integrating health economics into their strategies becomes increasingly critical to address the evidence demands for regulatory and reimbursement pathways, ensuring timely patient access to medical advancements.
Discussion
Clinical Research Organizations (CROs) are pivotal in advancing the field of medical devices, particularly when it comes to clinical trials. By partnering with CROss, manufacturers can benefit from expedited trial timelines, cost efficiencies, and enhanced data quality. This symbiosis is exemplified by the recent breakthrough designation awarded to a pioneering cervical cancer screening solution designed for home use.
The product's adherence to safety and efficacy standards underscores the importance of rigorous clinical trials in bringing life-saving technologies to market.
Despite these advantages, challenges persist in the CRO partnership landscape. For instance, a patient from rural Pennsylvania with an ultra-rare disease may face daunting logistics when a trial is conducted internationally, such as in Turkey. Similarly, discrepancies between the data medical device manufacturers submit to the FDA and the information payors require can lead to delays or denials in coverage, thereby hindering patient access to new devices.
To navigate these challenges, it is crucial to select and manage CRO partnerships meticulously, ensuring ethical conduct and data integrity. This approach aligns with the insights shared by digital healthcare experts at Human, who emphasize the significance of collaboration among diverse healthcare stakeholders to transform healthcare and improve patient outcomes. The collective aim is to mitigate complexities and streamline processes, which is essential for CROss to effectively contribute to the development and approval of medical devices.
References
Clinical research organizations (CROs) are pivotal in the landscape of medical device development. Their role encompasses not just the execution of clinical trials, but also navigating the intricate ethical, legal, and social issues that accompany the advancement of medical technologies. By considering factors such as market incentives and intellectual property, CROs contribute significantly to shaping the evolution of emergent medical devices.
Significant strides have been made in digital therapeutics, a novel form of digital health that employs software to treat medical disorders. These innovative therapies, which can be utilized via mobile devices, virtual reality, and sensors, offer promising avenues for managing chronic diseases and improving neurological outcomes. Despite their potential to decrease patient costs, digital therapeutics face challenges, such as limited insurance coverage and the need for more extensive clinical trial testing, which underscores the importance of CROs in this field.
The success of a new medical device project can often be determined right from its initial stages. It is crucial for all stakeholders, including key external parties, to align on project priorities, which typically revolve around budget, time, and quality. The foundational team meeting sets the tone, determining the hierarchy of priorities and establishing a shared vision.
Furthermore, clarity on roles and responsibilities, as guided by a RACI chart, is vital for a cohesive team effort.
In the regulatory realm, agencies such as the FDA in the United States categorize medical devices into classes based on risk, with class three devices like pacemakers undergoing the most stringent review processes. Understanding the regulatory landscape is essential for CROs as they guide medical devices from conception to market.
Moreover, the selection of target markets for Voice of Customer (VoC) research is a strategic step that cannot be overlooked. By analyzing disease incidence and prevalence data, CROss can identify representative locations for focused research. This approach, although not exhaustive, yields significant insights that are crucial for device development.
The United States stands as a global leader in pharmaceutical research, with investment leading to the management or eradication of diseases such as HIV and Hepatitis C. While the high cost of drugs is often justified by the need to fund future innovation, the importance of CROss in ensuring the effective and ethical development of medical technologies cannot be understated. Their role is not just to comply with regulatory demands but to actively enhance patient outcomes and contribute to a better healthcare system.
Conclusion
In conclusion, Clinical Research Organizations (CROs) are essential in the realm of medical device clinical trials. They offer specialized expertise and resources to navigate the complex journey from concept to market, ensuring safety and efficacy while overcoming regulatory challenges. CROs play a central role in the dialogue between innovation, regulation, and patient care, striving to advance medical device research and enhance patient outcomes.
CROs contribute to the growth of the medical device industry by offering end-to-end solutions, including development, manufacturing, and market entry strategies. Their ability to bridge the gap between innovation and practical application ensures that medical breakthroughs are accessible and implementable in real-world settings.
In conducting clinical trials, CROs employ innovative methodologies and processes to ensure efficiency and reliability. They navigate through protocol development, patient recruitment, data collection, and analysis while adhering to regulatory guidelines. Digital technologies and new approaches, such as Digital Clinical Trials (DCTs), enhance patient inclusivity and data collection efficiency.
The partnership between CROs and medical device companies revolutionizes the landscape of clinical trials, optimizing the reliability of results and forming diverse participant groups. CROs also address the logistical and ethical considerations of trials that span borders and involve diverse patient populations.
Despite challenges in the CRO partnership landscape, such as logistical issues and discrepancies in data submission, meticulous selection and management of partnerships ensure ethical conduct and data integrity.
In summary, CROs are instrumental in advancing the field of medical devices. Their contributions are crucial for the development and approval of medical advancements, and they play a critical role in transforming healthcare and improving patient outcomes. Through collaboration with diverse stakeholders, CROs strive to streamline processes, ensure ethical development, and enhance the effectiveness of medical technologies.




