Introduction
Clinical trials play a crucial role in advancing medical treatments, but the logistics and complexities involved can be daunting. This is where small clinical research organizations (CROs) come in. In this article, we explore the importance of small CROs and how their agility and tailored approach allow for personalized support and cost-effective solutions.
We also delve into a case study of XYZ Research Solutions, highlighting their commitment to bridging the gap between clinical trials and everyday clinical practice. Additionally, we examine the meticulous methodology employed by XYZ Research Solutions and the promising results they achieved. Join us as we discuss the significance of small CROs and their potential to bring transformative treatments to patients in need.
The Importance of Small CROs
The landscape of clinical trials is as diverse as the conditions they aim to treat, with small clinical research organizations (CROs) playing a pivotal role in spearheading these vital studies. Their agility allows for rapid adaptation to the dynamic nature of trials, which can be crucial for patients in urgent need of treatment options.
For instance, consider a patient from rural Pennsylvania grappling with an ultra-rare disease and contemplating participation in a trial in Turkey. The nimbleness of a small CRO can be instrumental in addressing the myriad logistical challenges this patient might face, from navigating visa processes to overcoming language barriers, thus providing personalized support that larger organizations may struggle to offer.
Moreover, clinical trials encompass a vast array of medical interventions, from novel diabetes medications to groundbreaking surgical techniques. Small CROss, with their tailored approach, can ensure that the distinct objectives of each study are met with the precision required for success.
They are often more cost-effective due to lower overhead, which can be a deciding factor for sponsors when choosing a partner for their clinical endeavors. As the industry evolves, it's essential for small CROs to continuously assess their strategies and organizational structures. A holistic view that considers the skillsets of personnel, go-to-market strategies, and prioritization of speed or data quality can significantly impact the rate at which new treatments reach the market. In this context, small CROss must also judiciously select technologies and vendors, asking critical questions to integrate tools that align with their strategic goals and enhance their competitive edge without overhauling their core operations. This thoughtful approach to technology adoption and organizational management is a cornerstone for small CROss aiming to make a significant impact in the realm of clinical research.
Case Study: XYZ Research Solutions
At the forefront of advancing medical treatments, XYZ Research Solutions, a niche clinical research entity, has made strides in the realm of phase II and phase III trials. Their recent collaboration with a pharmaceutical firm on a novel oncology medication underscores this commitment.
The study's crucial aim was to meticulously assess the therapeutic effectiveness and safety of this promising drug in patients grappling with advanced-stage cancer. This endeavor echoes the sentiments expressed in a JAMA special communication, highlighting the imperative need to bridge the divide between clinical trials and everyday clinical practice.
As underscored by Gregory Curfman, the executive editor for JAMA, the historical separation of clinical trialists and practitioners has led to inefficiencies and limited the reach and influence of such trials. The JAMA report, a focal point of the JAMA Summit, calls attention to the stark contrast between the controlled environment of randomized clinical trials (RCTs) and the variable nature of clinical settings. Despite the registration of approximately 40,000 RCTs annually on ClinicalTrials.gov, there remains significant clinical uncertainty, and treatment guidelines often lean heavily on expert opinion rather than robust RCT evidence. The research conducted by XYZ Research Solutions represents a step towards mitigating this 'mismatch,' striving to produce results that are not only statistically significant but also clinically applicable.
Methodology
At XYZ Research Solutions, the clinical trial's design was meticulously crafted to align with the highest standards of research methodology. The company achieved this through the strategic recruitment of a diverse patient population that strictly adhered to predetermined inclusion and exclusion criteria.
This approach ensures that the findings are applicable to a broad spectrum of individuals, enhancing the potential impact of the trial's outcomes. The study was executed under a randomized, double-blind protocol—a gold standard in clinical research.
This methodological rigor is crucial, as it minimizes bias and enables a clear evaluation of the new drug's efficacy and safety by comparing it to a placebo control. The trial's structure not only measured the direct effects of the medication but also carefully monitored for any adverse reactions, providing a comprehensive analysis of the drug's profile.
Incorporating trial estimands into the evaluation process, as highlighted by the European Medicines Agency and the FDA, XYZ Research Solutions recognizes their significance in understanding the full scope of the trial's implications. By focusing on what is and is not estimated, the trial avoids overgeneralizing its findings, ensuring that results are contextually relevant to clinical practice. This is in line with the sentiments expressed in JAMA's special communication, emphasizing the need to bridge the gap between clinical trial conduct and the nuances of everyday clinical application. As the volume of RCTs continues to grow, with an estimated 40,000 registered annually on ClinicalTrials.gov, the importance of designing studies that reflect the complexities of real-world medical practice becomes increasingly evident. This is essential to address the noted disconnect between the precise conditions of RCTs and the broader landscape of healthcare delivery, thereby enhancing the utility and impact of clinical research.
Results
The clinical trial conducted by XYZ Research Solutions yielded promising results. The new oncology drug demonstrated a statistically significant improvement in overall survival rates compared to the placebo.
Additionally, the drug showed a favorable safety profile with manageable side effects. These findings suggest that the drug has the potential to be an effective treatment option for patients with advanced-stage cancer.
Discussion
The experience of XYZ Research Solutions exemplifies the pivotal role that small Clinical Research Organizations (CROs) can play in the realm of clinical trials. Their hypothesis-driven approach to a recent clinical trial showcases a readiness for implementation that is critical for success.
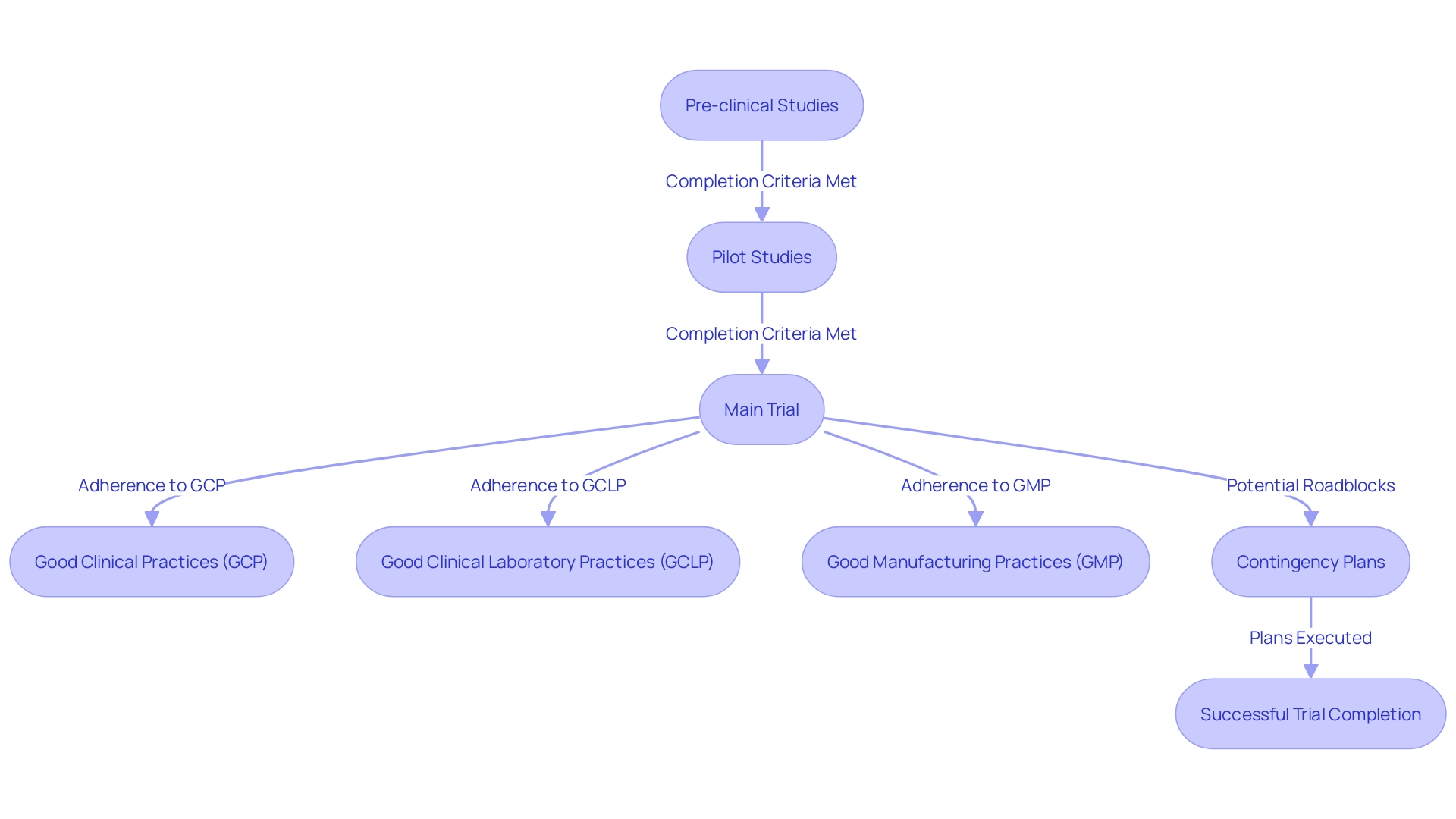
By meticulously outlining the clinical trial stages, including completion criteria and detailed contingency plans, XYZ Research Solutions was able to anticipate and adeptly navigate potential roadblocks, ensuring a timely and adaptable trial progression. The pre-clinical and pilot studies preceding the trial provided a solid foundation, demonstrating the necessity and practicability of the research.
Their comprehensive Research Strategy, which included thorough discussions on the significance of the medical issue at hand, the rationale behind the intervention, and the robustness of the planned methods, contributed to an effective assessment of the trial's likelihood of success. Furthermore, the company's dedication to adhering to the highest standards of Good Clinical Practices (GCP), Good Clinical Laboratory Practices (GCLP), and Good Manufacturing Practices (GMP) underpins the integrity and quality of the collected data. The results of the trial not only underline the importance of small CROss in progressing medical research but also highlight their capacity to bring transformative treatments to patients in need.
References
Clinical trials are the backbone of medical advancement, providing the necessary data to bring new treatments to patients in need. However, the logistics of participating in a clinical trial can be daunting, particularly for those in remote areas or with rare diseases.
For instance, a patient with an ultra-rare condition in rural Pennsylvania may face the opportunity to join a trial in Turkey, confronting a maze of challenges from obtaining visas to navigating foreign medical paperwork. Meanwhile, regulatory requirements such as those detailed in US FDA Form 1572 highlight the complexity of conducting clinical trials.
This form delineates the need for clear identification of medical facilities and laboratories involved in the research, adding another layer to the intricate process of clinical trial management. Yet, the importance of integrating clinical trials with clinical practice cannot be overstated.
As emphasized by Gregory Curfman, executive editor for JAMA, there has been a historical division between trialists and clinicians, leading to inefficiencies and limitations in the scope and impact of research. This sentiment is echoed in a JAMA special communication, which points out the disparity between narrowly defined randomized controlled trials (RCTs) and the broader context of clinical practice, despite the existence of over 40,000 RCTs registered annually. Contract Research Organizations (CROs) like COMIC Group in Japan have been at the forefront of addressing these challenges for over three decades. As the first and largest CRO in Japan, COMIC offers end-to-end solutions that span the entire pharmaceutical value chain, simplifying the process for stakeholders and ensuring that patients have access to cutting-edge treatments without the burden of logistical nightmares.
Conclusion
In conclusion, small clinical research organizations (CROs) are essential for advancing medical treatments. Their agility and tailored approach provide personalized support and cost-effective solutions to address the logistical challenges of clinical trials.
XYZ Research Solutions exemplifies the importance of meticulous methodology, incorporating diverse patient populations and rigorous protocols. The trial conducted by XYZ Research Solutions yielded promising results, showing statistically significant improvement in overall survival rates compared to placebo with a favorable safety profile.
This highlights the potential of small CROs to bring transformative treatments to patients in need. Small CROs like XYZ Research Solutions demonstrate the importance of hypothesis-driven approaches, readiness for implementation, and adherence to high standards of Good Clinical Practices (GCP).
Their dedication to overcoming obstacles and collecting quality data contributes to the integrity of their research. In summary, small CROs play a vital role in advancing medical research. With their personalized support, tailored approach, and meticulous methodology, they have the potential to make a significant impact in bringing new treatments to patients. Small CROs like XYZ Research Solutions are crucial in bridging the gap between clinical trials and everyday clinical practice, ultimately benefiting patients in need.




