Introduction
Clinical summaries serve as a vital tool in the realm of medical research, bridging the gap between complex studies and various stakeholders. These summaries provide a condensed and accessible synthesis of clinical trials, presenting key findings and outcomes in a form that is easily understood by healthcare professionals, regulatory bodies, and patients. By contextualizing research within ethical and practical frameworks, clinical summaries enable informed decision-making, enhance patient outcomes, and drive medical advances.
In this article, we will explore the definition, benefits, key elements, regulatory requirements, impact on patient engagement and trust, challenges in creating effective summaries, best practices for writing them, and future directions in clinical summary development. Join us as we delve into the world of clinical summaries and their crucial role in improving healthcare.
Definition and Purpose of Clinical Summaries
Clinical summaries serve as the bridge between complex medical research studies and the various stakeholders who rely on this information. These crucial papers combine the necessary discoveries and results of trials into a format that is more easily understandable by healthcare professionals, regulatory bodies, and individuals. To give an example, think about a trial investigating the effectiveness of chemotherapy drugs cisplatin and carboplatin in individuals with cervical cancer. The resulting medical overview would emphasize the noteworthy rise in patient survival rates when chemotherapy is administered before radiotherapy, in contrast to radiotherapy alone, thus offering valuable perspectives for medical practice.
Furthermore, the concise overviews are not only about displaying information; they are about improving comprehension. They use plain language to describe complex medical conditions and their implications, as demonstrated by the toolkit provided for stroke information, which explains symptoms and the nature of a transient ischaemic attack in terms easily grasped by non-specialists.
The significance of these documents is emphasized by the fundamental ethical frameworks that govern medical investigations, such as the Declaration of Helsinki and the Belmont Report, which promote the regard for participants and the allocation of advantages and hazards. Clinical synopsis exemplify these principles by guaranteeing the discoveries of investigation are conveyed distinctly and efficiently.
Within the domain of informatics in healthcare, these synopses utilize technology to enhance the administration of information in medical settings, advancing the objective of improving care for individuals. The incorporation of concise medical overviews into healthcare systems highlights their significance as a resource in directing well-informed decision-making and patient care results.
The advancement of medical synopses is also evident in the recent updates to guidance and toolkits that provide new examples and metrics to assist stakeholders in understanding the nuances of medical investigation. According to an authority, data gathered from trials is an indispensable resource in the field of life sciences, and synopses have a crucial function in utilizing this data for numerous vital choices in the sector.
To summarize, clinical synopses not only compress data but also place it in the moral and practical structures of healthcare, ultimately promoting effective decision-making and improving patient results.
Benefits of Clinical Summaries
Clinical synthesis are essential tools in medical investigation, providing a streamlined amalgamation of intricate studies. These synopses capture the core of the research, showcasing goals, approaches, findings, and potential constraints in a user-friendly format. They act as a connection between complex trials and healthcare professionals, assisting in rapid knowledge transfer and endorsing an evidence-based approach to care. The use of clinical summaries aligns with the PICO framework (Patient, Problem, Intervention, Comparison, Outcome) providing a structured approach to formulating clinical questions and summarizing evidence. For example, decision aids for individuals, which frequently come in the form of brochures or videos, utilize the PICO principle to clarify healthcare decisions, presenting options and their potential benefits and harms. Such aids are crucial in ensuring individuals make informed decisions aligned with their values, enhancing the consultation process with clinicians without supplanting it.
Clinical guidelines, when condensed into syntheses, serve as a compass for healthcare providers, offering a synthesis of evidence and expert opinion. However, it is acknowledged that these guidelines, while advantageous for standardizing care and reducing uncertainty, may not always encompass the individual's needs. The ever-changing nature of practice requires that these synopses not only encompass the condensed information but also maintain the adaptability to accommodate the distinct situations of every individual interaction. Recent insights from Medtronic, a global healthcare technology company, underscore the importance of innovative technologies in transforming patient care, highlighting the company's commitment to insight-driven care. The organization's wide range of expertise and the dedication to addressing complex health issues demonstrate the commitment to ongoing enhancement and patient-focused results that summaries in a medical context strive to assist.
Moreover, the incorporation of trial findings into synopses is vital for connecting the divide between investigation and application. This has been emphasized in discussions at the JAMA Summit, where the focus on trialists' and clinicians' efforts was highlighted as a means to overcome the siloed infrastructure that currently obstructs the transfer of studies into medical practice. As medical synopses develop to encompass the scope of medical investigation and direct decision-making, they not only promote openness and responsibility but also function as crucial instruments for advancing medical understanding and enhancing patient results.
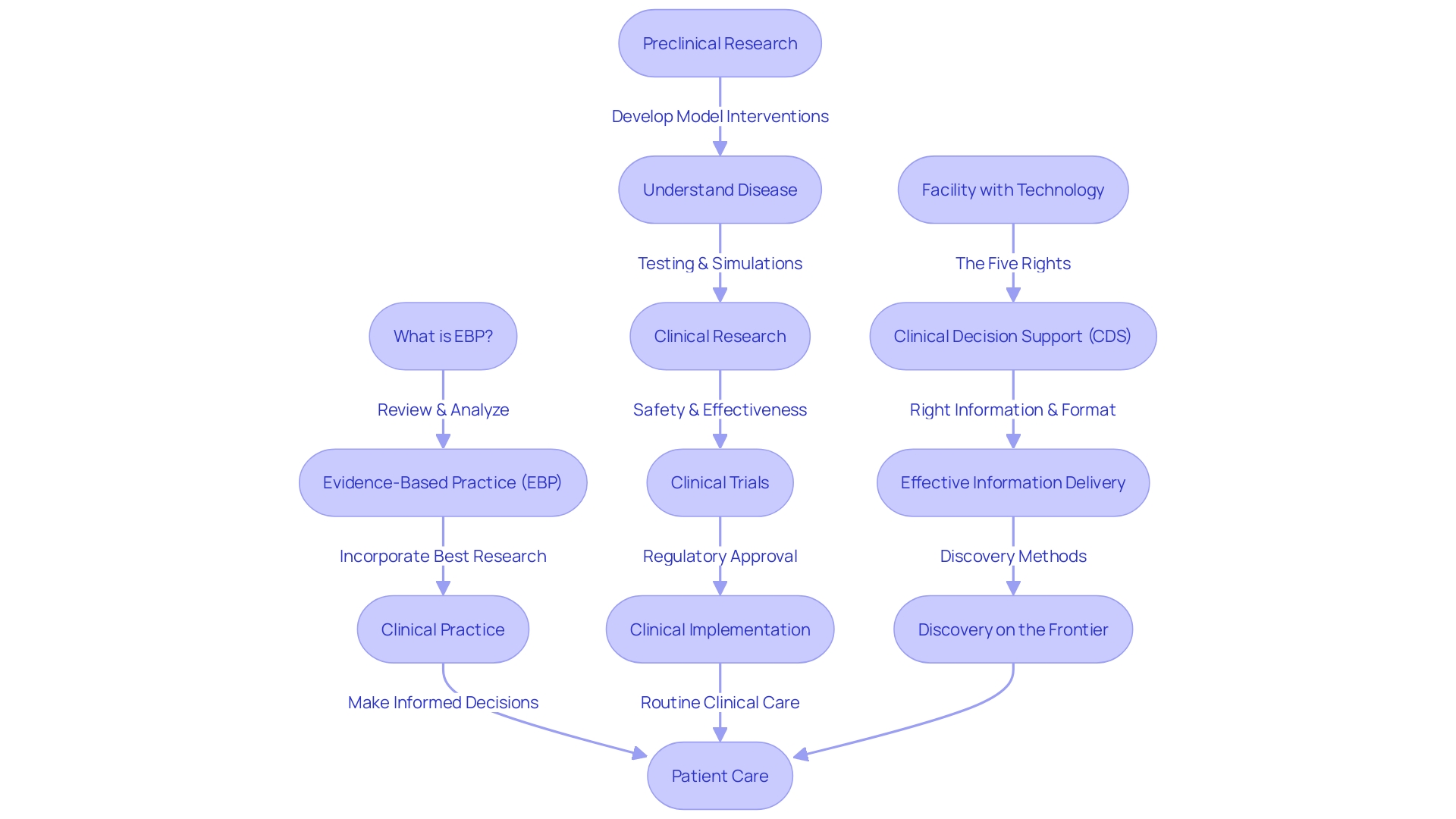
Key Elements of a Clinical Summary
A carefully prepared clinical synopsis is vital for succinctly expressing the core of medical investigation. It starts with a concise yet accurate explanation of the background, clarifying the main question or hypothesis. A comprehensive overview explores the intricacies of the study's design and methods, meticulously documenting how data was gathered, thereby providing a window into the investigation methodology. Key findings are condensed, providing clarity to the reader, and highlighting any outcomes of statistical significance or pertinence. It is crucial to take into account the study's restrictions and possible prejudices, guaranteeing a fair representation of the investigation. To sum up, the analysis may offer recommendations for future investigations or practical uses derived from the study's findings.
Clinical trials, such as those evaluating new treatments for lung cancer before official government approval, exemplify the endeavors in which clear, well-structured overviews are advantageous. These trials often explore novel combinations of approved drugs or new treatments for patients at different stages of their treatment journey. In this setting, a concise overview of the study must not only present the findings but also comply with ethical guidelines, such as providing transparent information on participant rights, defining susceptible populations, and outlining consent requirements for specific groups like pregnant women or prisoners.
Medtronic plc stands as a testament to the impact of medical investigation, with a mission to alleviate pain, restore health, and extend life. This global healthcare technology company, with a team of 95,000+ across 150 countries, embodies the drive to solve the most daunting health challenges. With technologies that impact two lives every second, every synopsis of clinical investigation contributes to this mission by elucidating the potential of new medical interventions.
When creating these synopses, it is recommended to use straightforward language to ensure clear and accurate communication, as intricate language might obscure the significance of the investigation. This aligns with the principle that summaries should be accessible to readers who, while perhaps familiar with rheumatological conditions, may not be versed in scientific jargon. The goal is to express what was accomplished, the main discoveries, how they enhance current understanding, and their potential impact on care.
The participation of individuals and family members in investigations design is progressively valued, reflecting a change towards study centered on individuals that produce more significant discoveries. This collaborative approach is backed by funders who acknowledge the importance of patient engagement in studies.
Basically, a concise overview functions as a connection between intricate study information and practical healthcare understandings, adding to the collaborative effort of enhancing wellness results globally.
Regulatory Requirements for Clinical Summaries
Maintaining the authenticity and accuracy of medical synopsis is crucial in the field of healthcare investigation. Regulatory bodies, like the U.S. Food and Drug Administration (FDA) and ethics committees, enforce particular guidelines that govern the preparation and dissemination of these synopses. These guidelines are created to uphold patient confidentiality, preserve the integrity of investigations, and maintain the ethical standards of medical studies. The fundamental aspect of these rules is the requirement for precision, openness, and dependability in the concise records, which are vital for the trustworthiness of the investigation.
ClinicalTrials.gov, for instance, showcases the dynamic nature of these guidelines, with its records evolving over the past 25 years to reflect changes in trial reporting laws and policies. As a result, the database now contains both required and non-compulsory data elements, offering comprehensive overviews of study protocols and findings. The modernization of the ClinicalTrials. Gov website further emphasizes the importance of transparent reporting by improving the way information is displayed and indicating what data was not provided by study sponsors and investigators.
Furthermore, recent FDA announcements, such as the final rule on 'Direct-to-Consumer Prescription Drug Advertisements', highlight the agency's commitment to clear communication of medical information. This rule mandates that major statements in drug ads be presented in a clear, conspicuous, and neutral manner, suggesting that the principles of clarity and transparency in regulatory requirements extend beyond summaries to include consumer-facing information.
The FDA's work to harmonize human subject protection regulations with the HHS Common Rule demonstrates an ongoing effort to streamline research while ensuring participant safety. This initiative is part of a broader commitment to advance well-designed clinical studies that provide the reliable data needed for informed decision-making about medical products. As researchers and organizations navigate the regulatory landscape, adherence to these evolving standards is critical for the generation of evidence that supports the safety and effectiveness of medical products, ultimately contributing to medical advances and the protection of public health.
Impact on Patient Engagement and Trust
Clinical synopsis play a crucial role in improving the involvement of individuals and strengthening the confidence within the healthcare network. These synopsis act as a link, transmitting intricate medical investigations in an approachable way, thus empowering individuals to form well-informed choices in collaboration with their healthcare practitioners. This empowerment through knowledge fosters active patient participation in their health management, leading to a deeper comprehension of their conditions and more confidence in their treatment options. Furthermore, the strategic communication of study contributions and the highlighting of collective efforts in study synopses can strengthen the bond of trust and perceived competence within the research community. Customized to cater to the distinct requirements of communities historically marginalized in biomedical studies, these synopses not only enhance health comprehension but also bolster the wider goals of precision medicine and health fairness. The incorporation of digital health technologies and commitment to optimal procedures in the development and distribution of these synopses guarantee they are a reliable and valuable educational resource, which plays a key role in the ongoing effort to enhance care for individuals and outcomes of investigation.
Challenges in Creating Effective Clinical Summaries
The process of creating concise overviews that communicate effectively to various readers requires condensing complex data into a comprehensible structure that maintains its scientific integrity. Researchers are tasked with the delicate equilibrium of embedding adequate detail while avoiding the alienation of readers through dense medical jargon. This precision ensures the integrity and dependability of the information, which is paramount. In addition, concise overviews must be tailored to the information requirements of diverse stakeholders, ranging from medical professionals to the individuals themselves. Using straightforward language, these overviews not only act as educational resources for participants keen to improve their understanding of health but also emphasize the importance of their contributions to scientific inquiry. Participation in clinical documents, like those from the All of Us Research Program, shows that when customized to tackle precision medicine and health equity, especially for communities historically excluded in studies, these papers can promote a feeling of mutual dedication and confidence.
For instance, the Report Card on prostate cancer treatments offers an exemplary model; it systematically delineates treatment definitions, survival rates, and personal anecdotes across its pages, culminating in a summation of a 'positive prognosis' and a repository of support services. Such synopses can be crucial in informing policy changes, as witnessed with the Peruvian Health Minister's consideration of the evidence from the Mamas del Rio program to potentially incorporate it into the national health system.
The challenge lies in ensuring that Plain Language Summaries (PLSs), which have historically made scientific papers more accessible to a non expert audience, continue to effectively communicate the core ideas of complex research. The advancement of medical knowledge dissemination necessitates that these synopses not only serve professionals within a field but also resonate with those outside of it, who may lack the foundational understanding assumed by traditional scholarly articles. By embracing open collaboration and leveraging advancements like external knowledge graphs and citation paper aggregation, researchers can enhance the summarization process, ensuring that it serves the evolving needs of the scientific and medical communities.
Best Practices for Writing Clinical Summaries
Creating clinical summaries that resonate with the intended audience requires more than just following a standard format—it demands a thoughtful approach that considers the needs and motivations of readers. For example, utilizing data from studies can raise awareness of participants' contributions to impactful investigation, improving their willingness to participate. Studies indicate that this approach also promotes a feeling of mutual dedication and reliance in the investigation procedure, which is vital for participants, especially those from marginalized communities in biomedical studies.
When creating these synopses, it's crucial to convey information in a straightforward, comprehensible language, converting synopses into valuable educational materials that can enhance health literacy. This is supported by a longitudinal dataset from the All of Us Research Program, which indicated that demographic subgroups are more likely to engage with health research overviews presented in an uncomplicated format.
Moreover, tailored health communication, which involves creating personalized content based on individual characteristics related to the desired outcome, has proven to be an effective method for promoting health behavior change. This aligns with the elaboration likelihood model, which posits that increased perceived personal relevance leads to more engagement and deeper processing of information.
Administrative tasks, such as writing medical visit notes, consume over half of healthcare providers' time, highlighting the need for efficient summarization processes. By giving attention to the most crucial elements of research - background, methods, key discoveries, and implications - researchers can produce synopses that not only fulfill the purpose of documentation but also improve the provision of healthcare.
Moreover, recent discoveries from a trial on cervical cancer highlight the significance of transparent communication in medical overviews. The trial disclosed that pre-radiotherapy chemotherapy enhanced patient survival rates, a crucial piece of information that must be accurately communicated in synopses for healthcare professionals and patients alike.
Hence, in order to produce efficient summaries in the medical field, scientists must not only conform to a standardized layout but also guarantee that the material is precise, dependable, and customized to meet the requirements of the intended audience. Ongoing interaction with stakeholders for feedback is crucial in improving the substance and user-friendliness of summaries related to medical treatment.
Future Directions in Clinical Summary Development
The field of clinical synopsis is on the verge of a change, propelled by advancements in technology and a more profound comprehension of individual requirements. In the field of stroke management, for example, having access to concise, simple explanations that outline symptoms and intervention strategies is essential. These synopses not only equip healthcare providers with quick insights but also function as critical tools for individuals encountering sudden and diverse symptoms, such as compromised mobility or communication.
Taking cues from the digital revolution in healthcare, companies like Medtronic are pioneering the integration of cutting-edge technologies into the healthcare sector. Through utilizing a worldwide network of experts and a variety of medical devices and systems, Medtronic's objective to enhance health outcomes is proof of the potential influence of technology on healthcare.
Generative artificial intelligence (AI) is one such innovation ready to redefine the production of medical overviews. With the capacity to process complex medical data and generate patient-centric content, AI can facilitate personalized care while addressing the concerns related to accuracy and ethical considerations. This is especially important in conditions like diabetes, where management is multifaceted and education is paramount. AI can distill vast amounts of data into actionable information, tailored to individuals' unique circumstances.
Moreover, the ethical implications of such technologies cannot be overlooked. As we traverse the legal and social terrains that these advancements occupy, it is essential to guarantee that the progress of concise overviews complies with the utmost principles of care and data management. The utilization of decision aids for individuals, which support them in making informed choices about their health interventions, exemplifies the combination of empowerment and technology.
In summary, the future of clinical summaries lies in the strategic integration of real-time data analytics, patient-reported outcomes, and AI-driven content creation. This evolution promises not only to streamline the delivery of healthcare information but also to place patients at the heart of their healthcare journey, fostering engagement and informed decision-making.
Conclusion
In conclusion, clinical summaries are essential tools in medical research, condensing complex studies into accessible formats that drive informed decision-making, enhance patient outcomes, and advance medical knowledge. These summaries streamline information transfer, promote evidence-based care, and uphold ethical standards.
Key elements of effective clinical summaries include precise articulation of research background, detailed study design and methods, distilled central findings, and consideration of limitations. Adhering to regulatory requirements ensures the integrity and validity of summaries, maintaining patient confidentiality and ethical standards.
Clinical summaries have a profound impact on patient engagement and trust, empowering individuals to make informed decisions and actively participate in their healthcare. Best practices involve using simple language, tailoring content to diverse stakeholders, and continuous engagement for feedback.
The future of clinical summaries lies in technological advancements and understanding patient needs. Innovations like generative AI offer potential for personalized content creation and streamlined information delivery. Integrating real-time data analytics and patient-reported outcomes will further enhance healthcare information dissemination and patient-centered care.
In summary, clinical summaries play a critical role in improving healthcare by condensing research data, providing actionable insights, and driving medical advancements. Embracing technology and patient-centered approaches will shape the future of clinical summaries, ultimately improving health outcomes worldwide.




