Introduction
Clinical research organizations (CROs) are instrumental in advancing medical research by providing expertise and support in clinical trial management. Specifically, CRO consultants play a crucial role in navigating the complex regulatory landscape and ensuring the efficiency and quality of research studies.
In this article, we will explore the significance of CRO consultants in medical research, the benefits of partnering with them, successful case studies, and the challenges they must overcome. By delving into these topics, we aim to shed light on the integral role of CRO consultants and their impact on the development of innovative treatments and medical interventions.
The Role of CRO Consultants in Medical Research
Clinical research organizations (CROs) play a crucial role in advancing medical research. These organizations provide support and expertise to pharmaceutical companies, biotech firms, and academic institutions in the design, execution, and management of clinical trials.
CRO consultants, in particular, bring specialized knowledge and experience to guide organizations through the complex regulatory landscape and ensure the efficiency and quality of research studies. Their contributions are vital in driving forward the development of new drugs, treatments, and medical interventions.
Benefits of Partnering with CRO Consultants
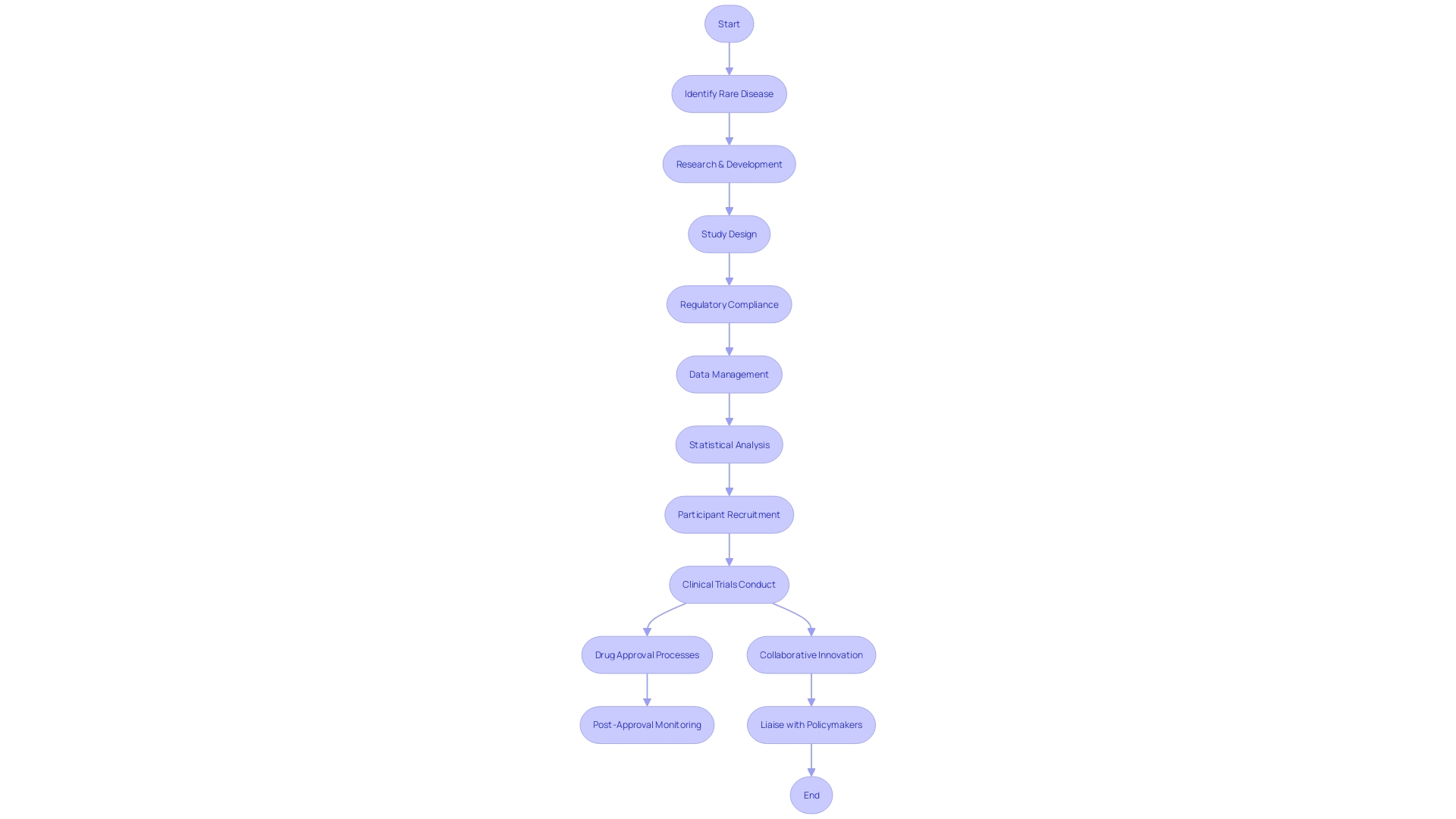
Clinical Research Organizations (CROs) are imperative for fostering groundbreaking medical research, particularly in scenarios involving ultra-rare diseases where established treatments are non-existent. Imagine a patient in rural Pennsylvania grappling with such a condition who is presented with the chance to join a clinical trial in Turkey. This represents not just hope for treatment but also the daunting task of navigating foreign travel and bureaucratic complexities.
Here, the role of CRO consultants becomes invaluable. They possess an intimate understanding of regulatory demands from authorities like the FDA and the EMA, which is instrumental in steering organizations through the maze of compliance and reducing approval bottlenecks. Additionally, their expansive networks of healthcare professionals, investigative sites, and research partners are crucial in expediting participant recruitment, ensuring diverse sample populations, and fostering collaborative innovation.
The expertise that CRO consultants bring in study design, data management, and statistical analysis cannot be understated. It heightens the scientific validity of studies, resulting in more authoritative and influential outcomes which, for a patient with a rare disease, could mean the difference between life and death. On top of this, they apply sophisticated project management techniques to coordinate complex research endeavors efficiently, effectively mitigating risks.
A prominent challenge in the field, as noted by industry experts, is the sheer number and uniqueness of rare diseases which necessitates precise and tailored measurement approaches. With thousands of conditions affecting minute populations, the scale and urgency for widespread knowledge are paramount. While funding for these studies has been historically limited, the shift towards more extensive, collective learning initiatives is an encouraging sign for the future of medical research and patient hope.
Case Study: Successful Implementation of CRO Services
CRO consultants play an instrumental role in optimizing the efficiency and effectiveness of medical research of critical importance to the advancement of treatments. One such example is when XYZ Pharmaceuticals ventured into the development of a pioneering therapy for a unique cancer type. Facing the multifaceted challenges of clinical trials, XYZ Pharmaceuticals sought the expertise of a CRO professional to facilitate every step of the process.
From crafting a meticulous trial protocol, ensuring stringent compliance with regulations, to selecting research locations and experts versed in the cancer subject matter, the CRO's guidance was indispensable. Their adept management of data and consistent oversight of trial conduct enabled high standards of research accuracy and protocol compliance. This thorough support accelerated the trial's completion, resulting in affirmative therapeutic effectiveness and swift regulatory endorsement, thus setting a high benchmark for the impact of CRO specialists in the realm of robust and innovative medical research.
Challenges Overcome and Lessons Learned
Collaborating with Contract Research Organization (CRO) consultants can bring about transformative benefits to clinical trials, such as regulatory compliance and enhanced scientific thoroughness. Nonetheless, forging a successful relationship with CROs entails navigating certain complexities.
Integral to this partnership is the establishment of effective communication and collaborative efforts. Ensuring that project goals, expectations, and milestones are consistently aligned demands clear and timely dialogue alongside the strategic use of project management tools.
Another layer of complexity arises from the potential for conflicts of interest, given that CRO consultants may have existing financial relationships with pharmaceutical companies or other entities within the clinical trial landscape. To circumvent such dilemmas, it is crucial to collaborate with CROss who prioritize integrity, transparency, and ethical standards.
Sound contractual agreements, coupled with strict adherence to industry benchmarks, are pivotal in mitigating any conflicting elements. The role of CRO consultants is indispensable in propelling medical research forward.
Their insights and proficiency play a critical part in the efficacious execution of clinical studies, which in turn fosters innovation and advances patient care. While there are challenges, an emphasis on open communication, ethical practice, and clear expectations will ensure that the collaboration with CRO consultants is optimized to its full extent. For instance, consider a patient from rural Pennsylvania battling an ultra-rare disease with no approved treatment. Presented with a chance to join a clinical trial in Turkey, the patient faces a labyrinth of logistical issues, such as visa procurement, language barriers with documents, and coordinating travel. Tailoring communications to address such real-world scenarios exemplifies the type of problem-solving required in clinical trial management, as exemplified by instructional practices used in firms like Nets, to render technical data more comprehensible and engaging.
Conclusion
In conclusion, CRO consultants play a vital role in advancing medical research by providing expertise and support in clinical trial management. Their contributions navigate the regulatory landscape, ensuring efficient and high-quality studies that drive the development of new drugs and treatments.
Partnering with CRO consultants brings significant benefits, including reducing approval bottlenecks and facilitating participant recruitment. Their expertise in study design and data management enhances the scientific validity of research, leading to more authoritative outcomes.
Additionally, their project management techniques effectively mitigate risks and coordinate complex endeavors. Successful case studies demonstrate the impact of CRO consultants in optimizing medical research efficiency and effectiveness.
Their guidance in clinical trial management accelerates the development of innovative therapies. This results in affirmative therapeutic effectiveness and swift regulatory endorsement, setting high benchmarks for CRO specialists' impact in robust medical research.
While challenges exist in establishing successful CRO partnerships, such as effective communication and managing conflicts of interest, their role remains invaluable. Clear dialogue, ethical standards, and adherence to industry benchmarks are crucial for maximizing collaborations. In summary, CRO consultants profoundly impact the development of innovative treatments. Their expertise and support enhance the scientific validity of studies, reduce approval bottlenecks, and accelerate the advancement of new therapies. By collaborating with CRO consultants, organizations can navigate research complexities and make significant strides in improving patient care.




