Introduction
The FDA's regulatory framework for medical devices is a complex system that manufacturers must navigate to bring their products to market. Understanding the nuances of device classification and registration pathways is essential for ensuring compliance and patient safety. This article provides a comprehensive overview of the FDA's 510(k) approval process, which involves determining the device's risk classification and choosing the appropriate registration pathway.
The article highlights the importance of substantial equivalence, where a new device is compared to an already legally marketed device, and discusses the benefits and challenges of the 510(k) clearance process. It also emphasizes the need for thorough documentation, a robust quality management system, and staying informed about FDA policies and updates. By adhering to the FDA's requirements and demonstrating substantial equivalence, manufacturers can successfully navigate the regulatory landscape and bring safe and effective medical devices to market.
Overview of the FDA's Regulatory Framework for Medical Devices
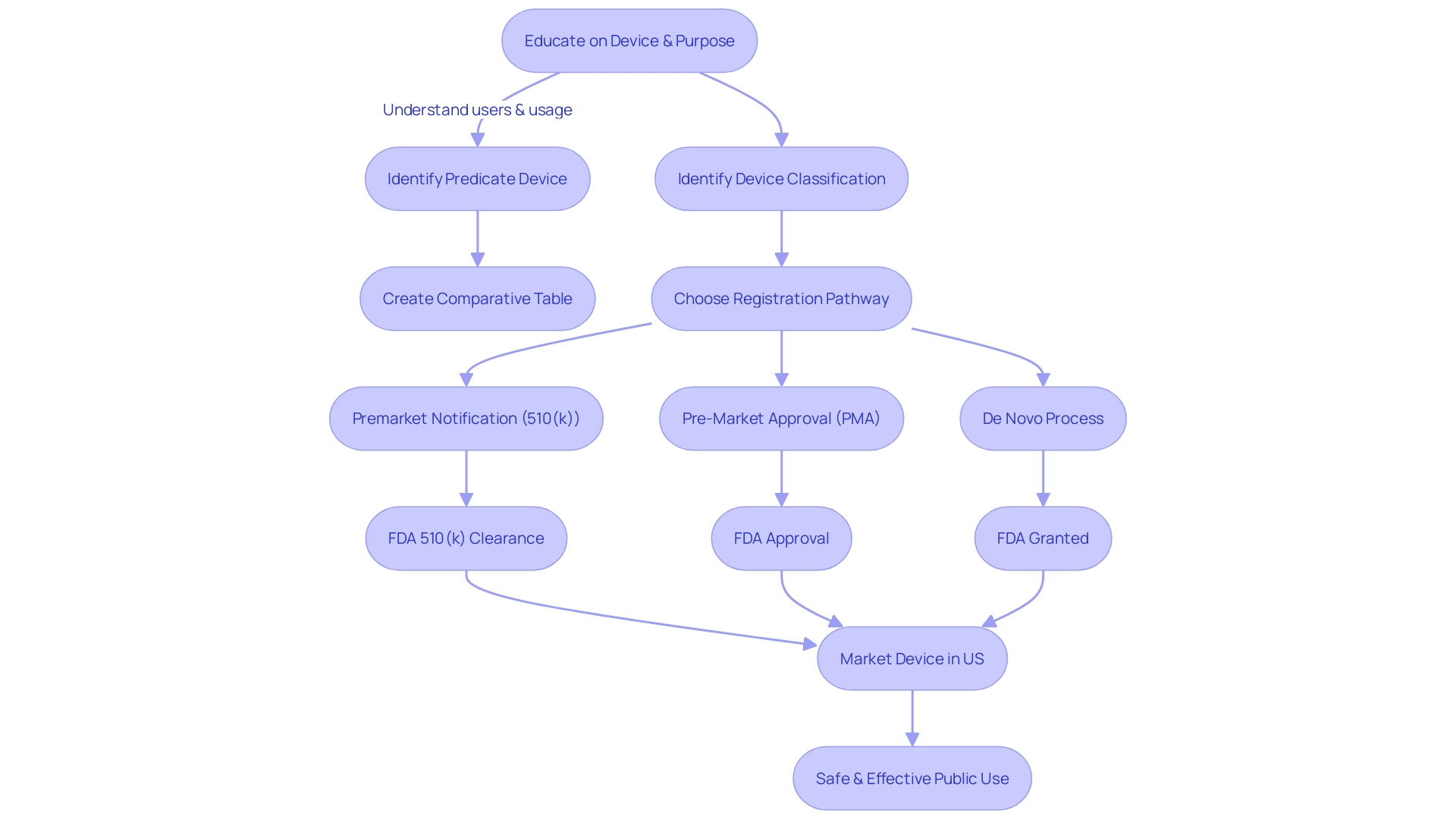
Manufacturers navigating the FDA's 510(k) approval process for medical devices must first determine the device's classification, which reflects the level of risk it poses to patients. The FDA categorizes devices into three classes, with Class I posing the least risk and Class III the most. The chosen pathway for registration, be it 510(k) Pre-Market Notification, Pre-Market Approval (PMA), or the De Novo process, is dependent on this classification.
To legally market a device in the U.S., it must be FDA Cleared, Approved, or, in the case of the De Novo process, Granted.
The 510(k) pathway allows for a more expedited entry to the market, as it requires demonstrating that a device is 'substantially equivalent' to an already legally marketed device. Notably, not all devices cleared through the 510(k) process require clinical trials, which was highlighted in the 2018 documentary 'The Bleeding Edge' when examining the clearance process and its implications for patient safety.
A strategic approach often employed involves routing novel technologies through existing regulatory pathways, which can facilitate quicker market entry and utilization of real-world experience to further applications of the technology. For example, the Apple Watch's heart monitor function was cleared by the FDA in 2018 for low-risk usage, which allowed the technology to be adopted swiftly and set a precedent for future innovative devices.
To enhance the evaluation and clearance process, the FDA encourages clear definitions of the intended use and risk assessment of models used in regulatory submissions. This involves articulating the specific questions or concerns the model addresses, the context of use, and the regulatory submission's informed decisions.
Public participation in this regulatory process is vital, and the FDA provides instructions for submitting comments. It is crucial for submitters to avoid including confidential information in their comments, as these will become public record. Those wishing to keep their information private must follow the specific procedures for written/paper submissions.
In conclusion, understanding the nuances of FDA classifications and registration pathways is essential for manufacturers. The FDA's nuanced approach to regulation, like the firm-based approach adopted after Apple's experience, aims to balance innovative medical device advancement with patient safety and efficacy.
What is a 510(k) Clearance?
Securing a 510(k) clearance from the FDA is a pivotal step for medical device manufacturers, signifying that their product is safe and effective for its intended use. This process involves a comprehensive review by the FDA to ensure that the new device is at least as safe and effective as a legally marketed device (predicate device) that is not subject to premarket approval.
The 510(k) clearance process is based on the principle of substantial equivalence, where a new device is compared with one or more similar devices already on the market. This comparison includes a detailed analysis of intended use, technological characteristics, and performance data, which may include clinical or non-clinical data, depending on the device's classification and the nature of its differences from the predicate.
For instance, the Vivio System by Ventric Health, which aids in the diagnosis of heart failure, is an example of a device that benefited from this process. As stated by the CEO of Ventric Health, early diagnosis of heart failure is critical, particularly for Medicare populations who are disproportionately affected. The Vivio System, which uses advanced algorithms to detect elevated left ventricular end-diastolic pressure, exemplifies the kind of innovative technology that can enter the market through the 510(k) pathway.
However, it's essential for manufacturers to understand that the 510(k) clearance is not without its challenges. Statements from Vivos regarding their device for sleep apnea treatment highlight the risks and uncertainties inherent in the process, such as potential regulatory scrutiny and the need for effective revenue strategies. Additionally, the FDA's classifications for medical devices carry different patient risk values, and choosing the correct classification is vital for determining the appropriate registration pathway.
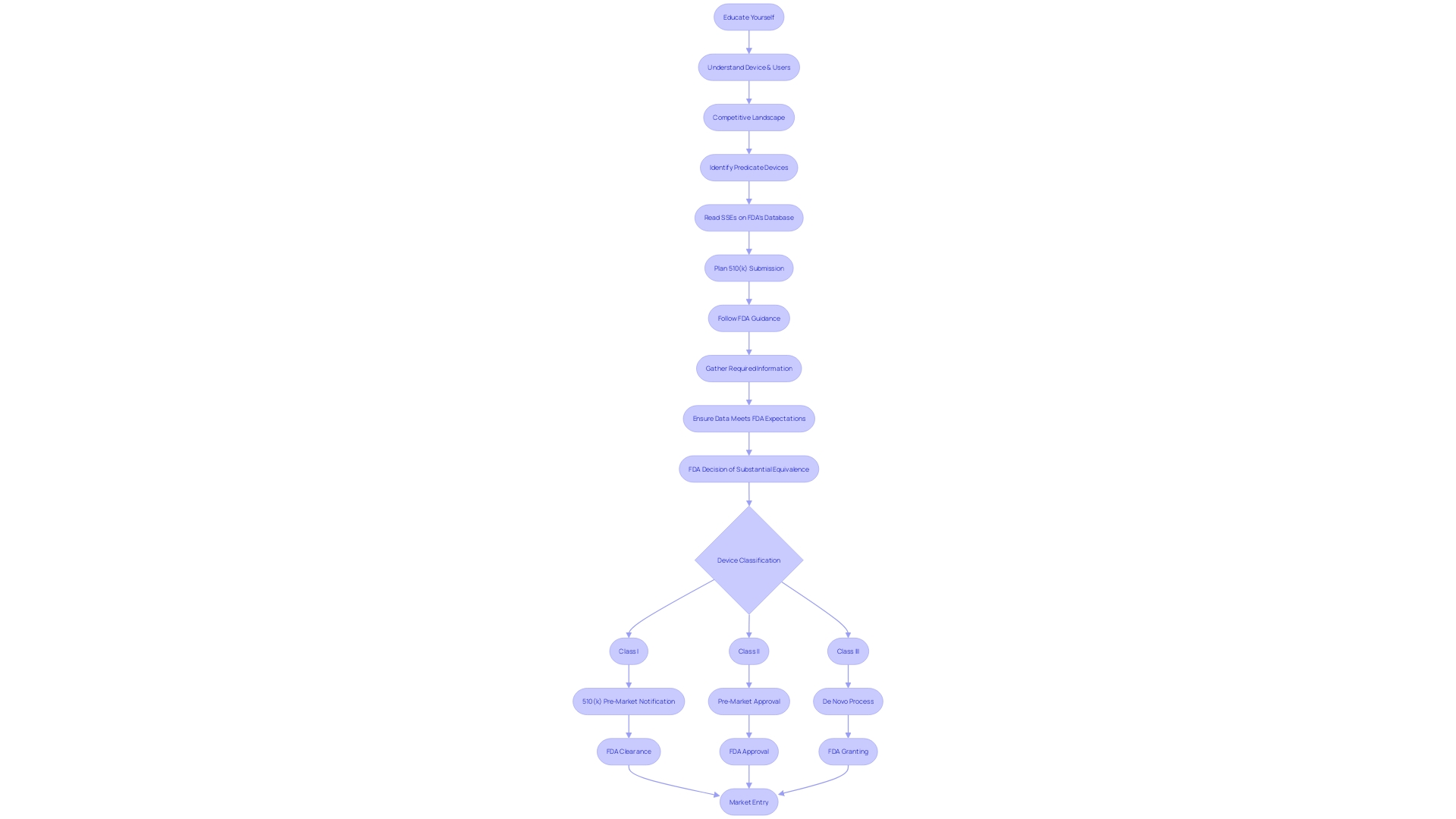
Educating oneself on the device's purpose, users, and the competitive landscape is crucial. By analyzing research literature, clinical studies, and existing devices' labeling, manufacturers can identify suitable predicate devices, which are central to creating a persuasive 510(k) submission.
The 510(k) clearance process has been under scrutiny, as evidenced by the 2018 documentary 'The Bleeding Edge,' which revealed that some devices were fast-tracked without the need for clinical trials, leading to patient injuries. This has prompted discussions about the need for legislative and regulatory reforms to ensure patient safety without stifling innovation in the medical device industry.
In conclusion, while the 510(k) clearance process offers a pathway for medical devices to reach the market, it is accompanied by significant responsibilities for manufacturers to thoroughly demonstrate substantial equivalence and address the inherent risks and uncertainties involved.
Purpose and Requirements of the 510(k) Process
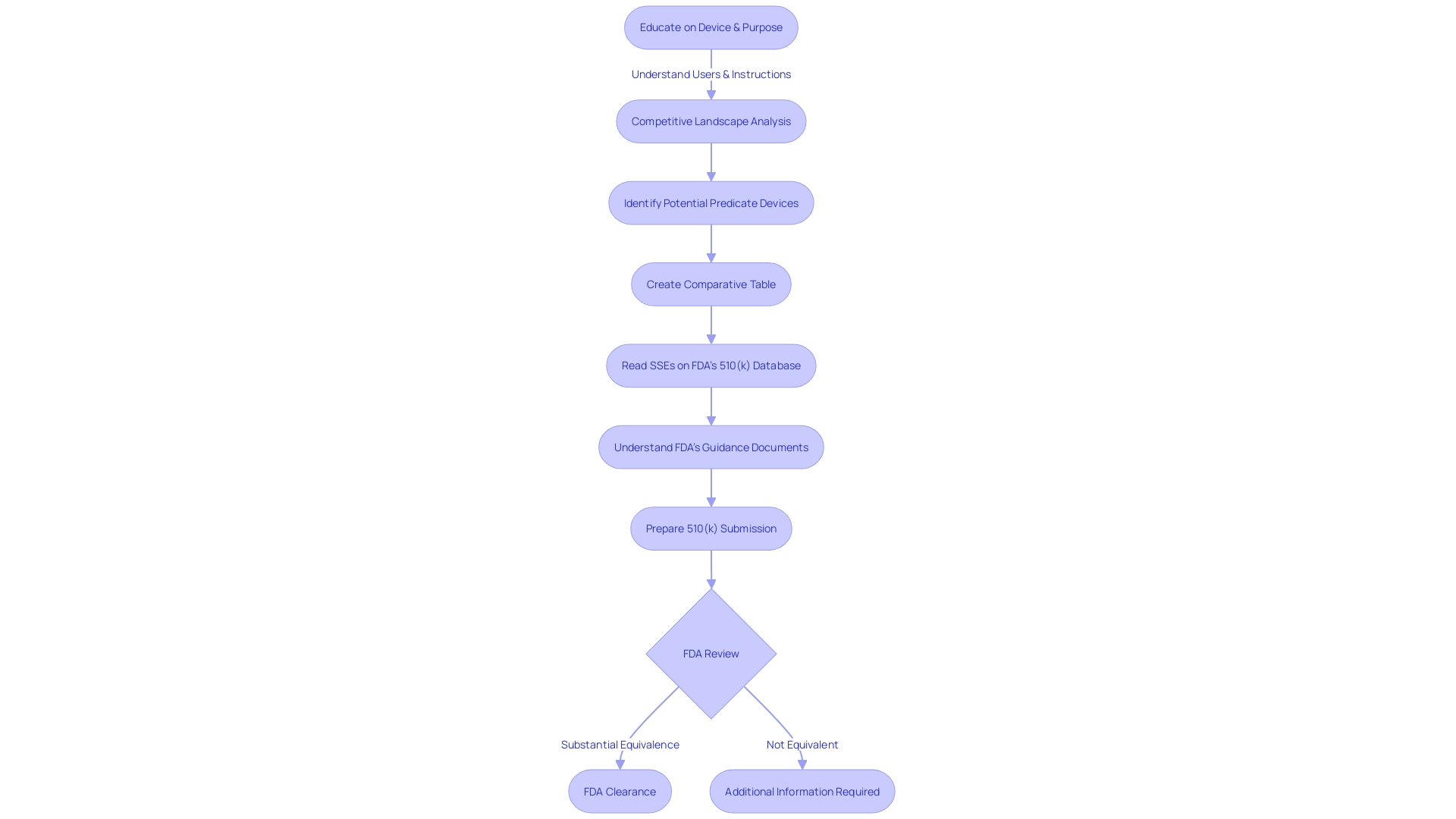
The 510(k) process, formally known as Premarket Notification, is a critical pathway for medical device manufacturers to obtain FDA clearance for marketing their products in the United States. It's imperative for manufacturers to grasp thoroughly the exact classification of the device, as the FDA categorizes medical devices into three classifications based on the level of risk they pose to patients.
Once the appropriate classification is determined, the manufacturer can proceed with the 510(k) submission, provided that their device is deemed substantially equivalent to a legally marketed device (predicate device) that does not require Pre-Market Approval (PMA). This substantial equivalence means that the new device must have the same intended use as the predicate and that it has the same technological characteristics or that any differences do not raise new questions of safety and effectiveness.
For the medical device community, understanding and differentiating the terms 'Registered', 'Cleared', 'Approved', and 'Granted' is essential, as they represent different FDA statuses, each with its unique implications for marketing and regulatory obligations. 'Cleared' specifically refers to the status granted to a device that successfully undergoes the 510(k) process, signifying that the FDA has reviewed the device and determined it is safe and effective for public use based on its substantial equivalence to a predicate.
Recent scrutiny, such as the insights from the 2018 documentary 'The Bleeding Edge', has highlighted that not all devices undergoing the 510(k) process are mandated to undergo clinical trials, which can lead to concerns about patient safety. The FDA remains vigilant in its responsibility to assure the safety and effectiveness of medical devices, which also involves updating and refining regulatory pathways in response to technological advances and public health needs.
To navigate the 510(k) process successfully, a deep understanding of the device, its users, and the competitive landscape is crucial. Manufacturers should conduct comprehensive research, including reviewing clinical studies, competitor devices, and market data, to build a comparative table that clearly distinguishes their device from existing products.
Moreover, staying informed about the latest FDA policies, such as the anticipated updates to the Breakthrough Devices Program and the implications for digital health technologies, is crucial for staying ahead in the rapidly evolving regulatory environment. This proactive approach ensures that manufacturers align with the FDA's mission to protect public health while fostering innovation in medical device development.
Regulatory Pathways for Evaluating Medical Devices
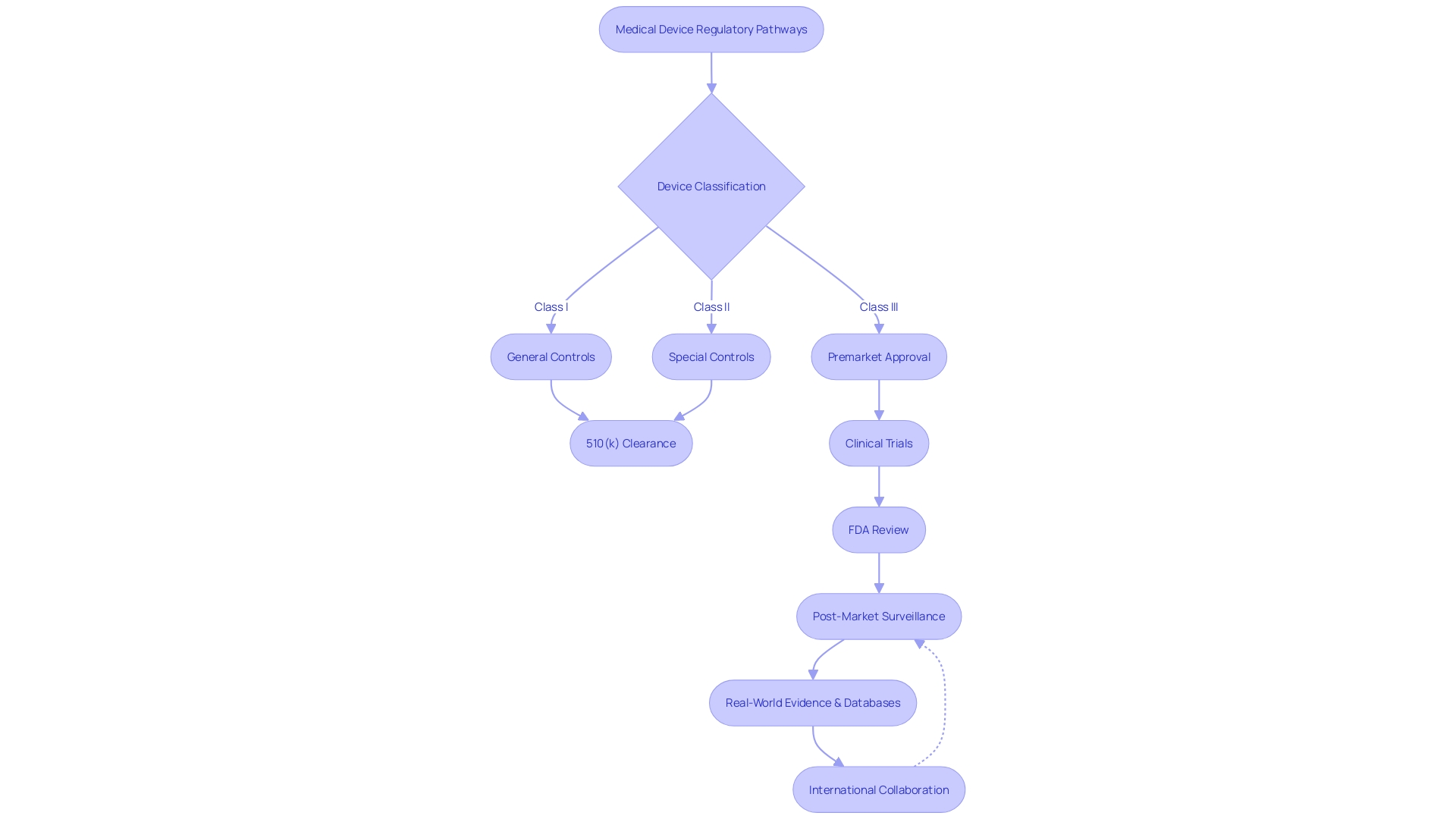
The regulatory landscape for medical devices is complex and requires careful navigation. The FDA's classification of medical devices into three distinct classes based on potential risks underscores the importance of manufacturers understanding which regulatory pathway to pursue. For Class I and II devices, which include lower-risk products, the 510(k) clearance process is commonly utilized.
This process entails comparing the new device to an existing, legally marketed predicate device, allowing for a more streamlined entry to the market if substantial equivalence is established.
In contrast, Class III devices, which encompass high-risk products crucial for sustaining life, such as pacemakers, are subject to more stringent scrutiny. They represent a smaller portion, approximately 10% of medical devices, but require a more rigorous approval process due to their critical nature. The goal of the approval process is not only to bring medical devices to market but to ensure that they are safe, effective, and of high quality, in accordance with CGMP requirements.
Recent efforts to improve regulatory pathways have included international collaboration, aiming to reduce approval times for devices that fill unmet medical needs. This has been evident in the MHRA's proposed regulations, which prioritize patient safety and aim for greater harmonization with international standards. The MHRA's statement of policy intent for international recognition of medical devices is a move towards more patient-centered requirements, reflecting a commitment to innovation and the expedited delivery of transformative health technologies.
Real-world evidence, such as the case of the heart monitor in the Apple Watch, demonstrates the FDA's flexibility in adapting regulatory policies to accommodate new technologies. Moreover, the use of databases like MAUDE, augmented by analytical tools like Device Events, helps stakeholders monitor post-market device performance, ensuring ongoing patient safety and compliance.
As regulatory bodies continue to evolve their policies, manufacturers must remain vigilant, informed, and engaged with the changing requirements to ensure their devices meet the necessary standards and provide the greatest benefit to patient health.
Substantial Equivalence: The Core Principle of 510(k) Clearance
At the heart of the 510(k) clearance pathway is the concept of substantial equivalence. It is a pivotal criterion that medical device manufacturers must satisfy to navigate the FDA's approval process successfully. Substantial equivalence signifies that a new medical device is as safe and effective as a legally marketed predicate device not subject to Premarket Approval (PMA).
Understanding this principle is crucial for manufacturers since it directly impacts the FDA's decision on whether a device can enter the U.S. market.
To demonstrate substantial equivalence, manufacturers must meticulously compare their new device to one or more similar devices (predicates) already on the market. This comparison includes a detailed analysis of intended uses and technological characteristics. If the new device has the same intended use as the predicate and the technological differences do not raise new questions of safety and effectiveness, it is generally deemed substantially equivalent.
The FDA provides clear scenarios where clinical data may be required to establish substantial equivalence. These instances might include variations in the device's indications for use, technological differences that could affect safety or effectiveness, or when non-clinical testing is insufficient to demonstrate equivalence. Additionally, if there is new or increased risk associated with the predicate device, clinical data may be necessary to ensure the safety and efficacy of the new device.
Manufacturers must stay abreast of regulatory updates and public comments that could influence the 510(k) process. It is imperative to protect sensitive information when submitting comments to public dockets, ensuring that confidential data remains undisclosed.
In the ever-evolving landscape of medical device regulation, a comprehensive understanding of the device, its use, and the competitive environment is essential. This includes researching literature, clinical studies, and competitor information to support the 510(k) submission. With the FDA's mission to approve innovative drugs and devices that bring new treatment options and advance healthcare, manufacturers are encouraged to align their submission strategies with the FDA's guidance to facilitate a smoother approval journey.
Steps Involved in the 510(k) Submission Process
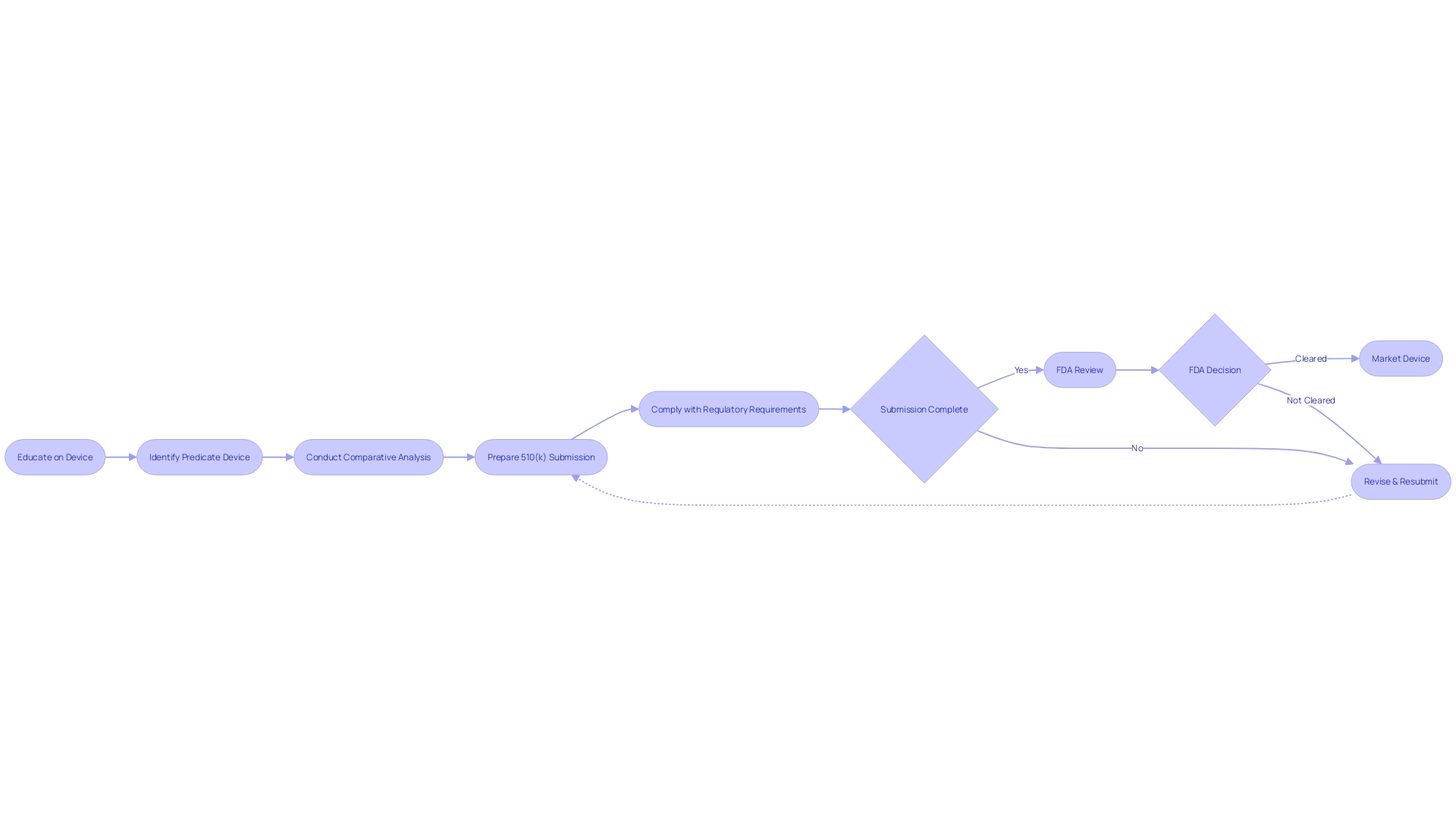
Navigating the 510(k) submission process is a complex task that involves meticulous planning and thorough documentation. Manufacturers must begin by gaining an extensive understanding of the medical device in question, considering its intended users—such as clinicians, physicians, dentists, or patients—and studying the instructions for use, including all warnings and precautions. The identification of a predicate device, which has the same intended use and similar technological characteristics, is a fundamental step.
This involves a detailed review of research literature, clinical studies, and competitor information to establish a comparative analysis.
Once the groundwork of comparative analysis is set, the next critical phase is the preparation of the 510(k) submission itself, which must be comprehensive, containing detailed information across all facets of the device's development and testing. This is crucial, as the FDA's decision-making hinges on the accuracy and completeness of this documentation. The FDA's categorization of medical devices into three classes—ranging from low-risk class one to high-risk class three—dictates the level of regulatory scrutiny a device will undergo.
Class one and two devices are generally subject to the 510(k) clearance process, which involves comparison to a predicate device.
However, it is essential to note that not all devices require clinical trials for clearance. This was highlighted in a 2018 documentary that shed light on the FDA's clearance process, revealing that some devices can be fast-tracked based on their similarity to existing products. This aspect of the process has been subject to scrutiny, especially in cases where devices led to patient injuries.
Ensuring that the submission does not contain any confidential or personal information that should not be made public is also a critical component of compliance.
The journey from pre-submission to the final stages of the process is arduous and often extends over several months, with manufacturers needing to compile, review, and format a vast amount of information. The experiences shared by the founders of Artos, who have navigated life sciences' regulatory submissions processes, underscore the importance of an organized and informed approach to the 510(k) submission. Their insights reveal the necessity of an integrated strategy that encompasses a deep understanding of the regulatory environment, competitive landscape, and the rigorous expectations of health agencies like the FDA.
Documentation and Quality Management System (QMS) for 510(k) Submission
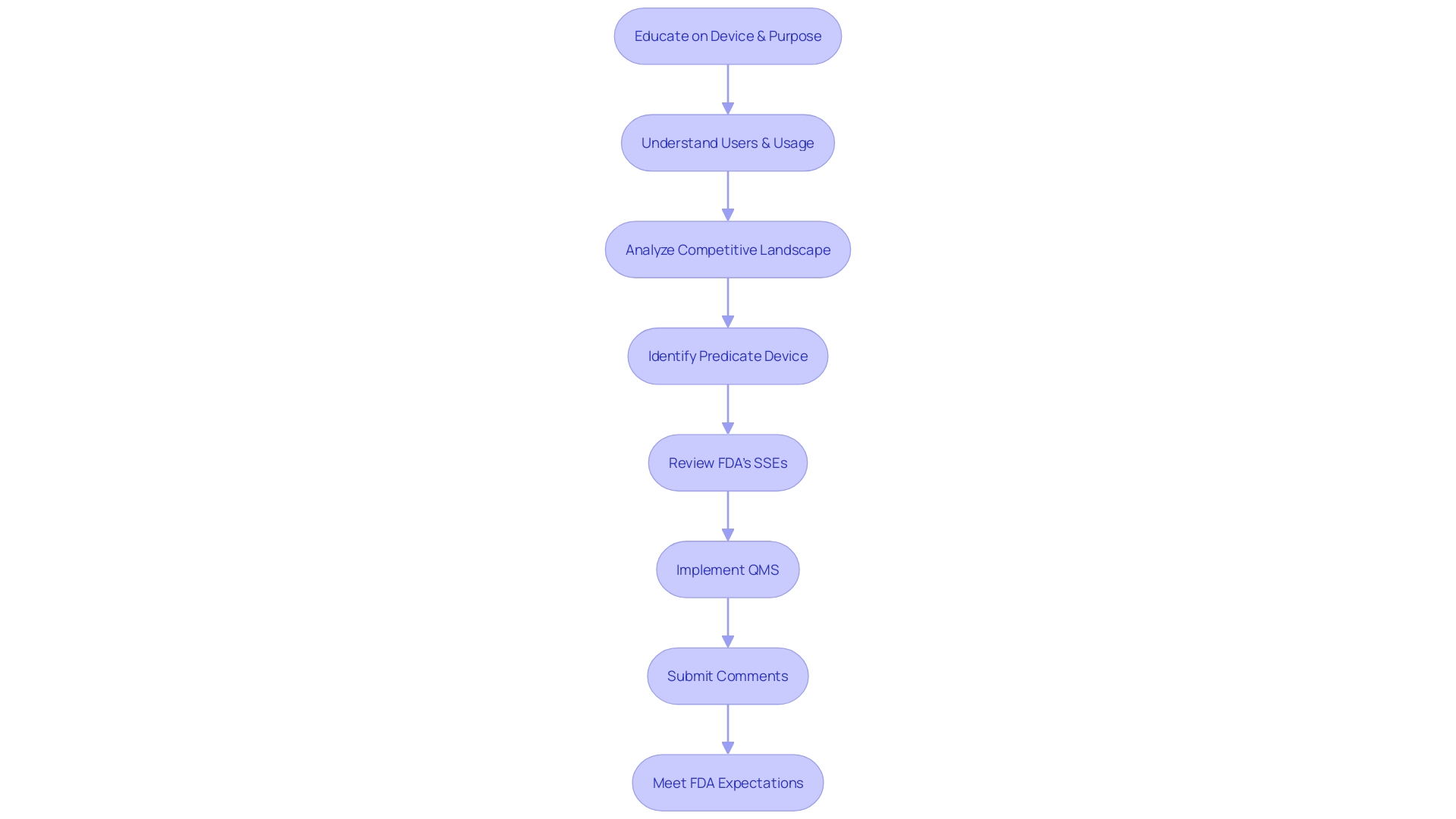
A well-prepared 510(k) submission is underpinned by meticulous documentation and a robust quality management system (QMS). To achieve compliance and facilitate a smooth approval process, it is vital to garner a comprehensive understanding of the medical device, including its intended users such as clinicians, physicians, dentists, and patients, as well as the detailed instructions for use, complete with any warnings and precautions.
In collaboration with the Marketing team, it is also crucial to examine the competitive landscape, focusing on devices similar to the one you wish to bring to market. Through a thorough analysis of research literature, clinical studies, and marketing materials of competitor devices, you can begin to construct a comparative table. This table will help identify a predicate device that shares the same intended use and technological characteristics as your device.
Once a suitable predicate device is identified, it is essential to review the Summaries of Safety and Effectiveness (SSEs) found in the FDA's 510(k) database. This evaluation of similarities and differences will be a cornerstone of your 510(k) submission.
The FDA's guidance outlines a comprehensive QS model that aligns with the current good manufacturing practice (CGMP) regulations for manufacturing human and veterinary drugs, including biological drug products. By implementing a modern QMS and risk management approaches that meet these regulations, manufacturers can ensure full compliance.
Furthermore, it is imperative to be mindful when submitting comments to the FDA, especially electronically. Any confidential information, such as medical data or Social Security numbers, should not be included in comments as they will become public records. For confidential submissions, a written/paper format is recommended.
A clear understanding of the FDA's expectations through their guidance documents, and a strategic approach to compiling the necessary information, is essential. The challenge is to collect all required data within the allotted timeline, ensuring that the 510(k) submission will result in a decision of substantial equivalence by the FDA. This preparation is not just about compliance but also about ensuring the safety and effectiveness of medical devices entering the market.
Examples of Devices That Require 510(k) Clearance
While not all medical devices necessitate 510(k) clearance, the FDA does require it for a significant number of devices, particularly those that are not exempt and which may pose a risk to patients if not properly evaluated. To determine if a device requires 510(k) clearance, manufacturers must understand the classification of their product and whether it meets the criteria set forth in section 201(h) of the Federal Food, Drug, and Cosmetic Act. For example, the Impella Connect System, which includes software and hardware components to support heart function in critical care, underwent premarket notification due to its sophisticated alarm system and remote monitoring capabilities.
The 510(k) clearance process came under scrutiny in the 2018 documentary 'The Bleeding Edge,' which highlighted instances where fast-tracked devices led to adverse patient outcomes, including bleeding and organ puncture. Despite these risks, clinical trials are not always mandatory for 510(k) clearance, raising concerns about the thoroughness of the premarket evaluation.
In recent news, companies like OxiWear have successfully navigated the 510(k) process, receiving clearance for devices that monitor health conditions such as hypoxemia. The clearance is a testament to the rigorous testing and development these companies undertake to ensure safety and efficacy, as emphasized by Shavini Fernando, CEO of OxiWear. Dr. Panagis Galiatsatos from Johns Hopkins University Hospital noted the life-saving potential of such devices.
The FDA's commitment to fostering innovation while ensuring safety is evident in the recent clearance of devices designed to improve the quality of life for people with chronic diseases. The agency's efforts to clarify guidance, as seen in the Breakthrough Devices Program, demonstrate an ongoing evolution aimed at supporting advancements in medical technology.
Understanding the 510(k) process is critical for manufacturers. It's advised to identify potential predicate devices with the same intended use and similar technological characteristics to create a comparative table, as suggested by experts. This involves reviewing a vast array of content, including research literature, clinical studies, and FDA's 510(k) database summaries, to evaluate similarities and differences with existing devices.
In conclusion, the 510(k) clearance process is a complex and vital step for medical device manufacturers. It requires a thorough understanding of the FDA's regulations and a detailed assessment of the device's safety and effectiveness, all while keeping in mind the potential impact on patient care and the healthcare system at large.
When a Device Is No Longer Eligible for 510(k) Clearance
Medical devices that no longer meet the criteria for 510(k) clearance due to various factors such as safety concerns or design changes require alternative pathways for approval. As the FDA continues to refine its evaluation of devices, manufacturers must navigate this complex landscape. The FDA's recent guidelines emphasize the need for manufacturers to avoid using predicate devices with a history of recalls or unresolved safety issues in their 510(k) submissions.
This is crucial, as devices cleared through this process are diverse, including X-ray machines and wheelchairs. The FDA asks for detailed justifications when a predicate with a recall history is used, ensuring the new device rectifies previous issues.
Understanding the different FDA terms is vital. The FDA defines 'restricted device' as one with sale, distribution, or use limitations imposed by regulation or premarket approval conditions. Identifying the correct classification of a device is the first step, followed by choosing the appropriate registration pathway—510(k), PMA, or De Novo.
The terms 'Registered', 'Cleared', 'Approved', and 'Granted' reflect the status of a device within the FDA's regulatory framework, each signifying different levels of compliance and market readiness.
The documentary 'The Bleeding Edge' highlighted the fact that many medical devices do not require clinical trials before receiving 510(k) clearance, raising concerns about patient safety. The FDA's active postmarket surveillance system, designed to detect medical device safety issues, plays a critical role in ongoing oversight. However, challenges such as funding and patient identification have hindered the full implementation of this surveillance system.
Despite these efforts, a 2018 study linked over 1.7 million injuries and 83,000 deaths to medical devices in a decade, underscoring the importance of thorough premarket evaluation and post market monitoring.
Benefits and Importance of 510(k) Approval for Medical Devices
Securing 510(k) clearance is a pivotal step in ensuring medical devices are safe and effective for public use. It serves as a confirmation that a new medical device is substantially equivalent to a previously legally marketed device, facilitating its entry into the healthcare market. The clearance process, governed by the FDA, necessitates a meticulous comparison of the new device to an existing predicate, including a review of intended use and technological characteristics.
This comparison is crucial as it reassures healthcare professionals and patients alike that the device meets the stringent safety and efficacy standards set forth by the FDA.
The relevance of 510(k) clearance extends beyond safety into the competitive landscape of medical devices. Manufacturers must possess a thorough understanding of their device, including its operation, user instructions, and any potential risks or warnings. Additionally, in-depth knowledge of the market and competitor devices is imperative, enabling companies to position their product effectively.
Through this comprehensive approach, manufacturers are better equipped to navigate the regulatory environment, thus increasing the likelihood of successful market entry and adoption by healthcare providers.
Understanding the FDA's classification system is also essential. Devices are categorized into three classes based on the level of risk they pose to patients, with Class I representing the lowest risk and Class III the highest. The classification determines the regulatory pathway, be it 510(k) clearance, Pre-Market Approval (PMA), or the De Novo process.
Clarity on these terms is vital for regulatory professionals, as each classification and corresponding pathway represents different levels of scrutiny and requirements for market authorization.
With approximately 10% of medical devices falling under the high-risk Class III category and requiring more extensive review processes, it's clear that most devices are subject to the less stringent 510(k) clearance. Nevertheless, manufacturers must be diligent in their pursuit of FDA clearance, as the process not only affirms the device's comparability to existing solutions but also its readiness to improve patient outcomes and enhance the quality of life.
Conclusion
In conclusion, navigating the FDA's regulatory framework for medical devices requires a thorough understanding of device classification and registration pathways. The 510(k) approval process is a crucial step in bringing safe and effective medical devices to market. By demonstrating substantial equivalence to an already legally marketed device, manufacturers can obtain FDA clearance.
Thorough documentation and a robust quality management system (QMS) are essential for a successful 510(k) submission. Manufacturers must conduct comprehensive research, review clinical studies, and stay informed about FDA policies. By adhering to the FDA's requirements and demonstrating substantial equivalence, manufacturers can navigate the regulatory landscape and ensure compliance and patient safety.
The 510(k) clearance process offers a streamlined entry to the market, but it is not without challenges. The process has faced scrutiny, with concerns raised about devices being fast-tracked without clinical trials, leading to patient injuries. Manufacturers must address these risks and uncertainties and thoroughly demonstrate substantial equivalence.
Understanding the FDA's classification system is crucial, as it determines the appropriate regulatory pathway. Manufacturers must carefully evaluate their device's classification and choose the right pathway for market authorization. By doing so, they can ensure the safety and efficacy of their devices while fostering innovation in the healthcare industry.
Overall, the 510(k) clearance process plays a vital role in bringing medical devices to market. By following the FDA's regulations, manufacturers can navigate the complex regulatory landscape and contribute to patient safety and well-being. It is essential to stay informed about the evolving regulatory environment and prioritize compliance and quality throughout the development and approval process.




