Introduction
Form FDA 1572, known as the Statement of Investigator, is a cornerstone document in clinical research, ensuring that investigators are qualified and committed to maintaining regulatory standards throughout the conduct of clinical trials. This article delves into the multifaceted role of Form FDA 1572, emphasizing its purpose in safeguarding participant rights and adhering to investigational plans as stipulated by the Code of Federal Regulations (CFR). It explores who must complete the form, detailing the responsibilities of the principal investigator and key research personnel.
Additionally, the article outlines the critical information required on the form, such as investigator credentials and study descriptions, and highlights the investigator’s responsibilities, including protocol adherence, adverse event reporting, and data protection. The submission process and the severe consequences of non-compliance are also examined, underscoring the importance of timely and accurate documentation. Special considerations for foreign clinical studies are discussed, focusing on the necessity of maintaining ethical and scientific standards across diverse regulatory environments.
Purpose of Form FDA 1572
Form FDA 1572, referred to as the Statement of Investigator, plays a crucial role in medical research. This document guarantees that researchers have the required qualifications to perform clinical studies, aligning their work with regulatory standards. The form outlines the researcher's commitments and responsibilities, including adherence to the investigational plan and protecting the rights, safety, and welfare of participants under their care as stipulated by § 312.60 of the CFR. By signing the 1572 form, researchers confirm their understanding of the study requirements, including the necessity of informed consent, which must be presented clearly to facilitate participants' comprehension of the research, its risks, benefits, and procedures. This step is essential for preserving the integrity of research studies, which are vital in evaluating the safety and effectiveness of new therapies and interventions. The FDA, as part of the U.S. Department of Health and Human Services, underscores the importance of these measures in safeguarding public health and advancing medical research.
Who Must Complete Form FDA 1572?
The lead researcher (PI) holds the main responsibility for finishing Form FDA 1572 in a clinical study. This form must be meticulously completed by anyone designated as a researcher in studies involving investigational drugs or biologics. The form ensures that all researchers are thoroughly documented and accountable, addressing safety concerns and compliance with regulatory standards. Co-investigators and other key research personnel may also need to provide their details on this form. Proper documentation is crucial, as inadequate records can lead to significant issues such as increased medical errors and negative patient outcomes. According to the FDA, maintaining accurate and comprehensive documentation is essential for safeguarding public health and ensuring the efficacy and safety of medical products. This meticulous approach to documentation impacts not only the validity of the clinical trials but also their generalizability and long-term insights into participant health outcomes.
Information Required on Form FDA 1572
Form FDA 1572 requires extensive information about the researcher and the study site. Essential components of the form consist of the researcher's qualifications, contact information, and the names of any co-researchers. It also requires a detailed description of the study, encompassing its objectives, the investigational product, and any potential risks to participants. This comprehensive data collection is crucial for regulatory oversight, which ensures the safety, effectiveness, and security of medical products. The FDA, an agency within the U.S. Department of Health and Human Services, plays a vital role in this process by protecting public health. The agency's guidelines assist in upholding transparency and compliance with safety protocols in research. Documentation of adverse events and the decision-making processes surrounding reportable events are key aspects of maintaining compliance with regulatory standards.
Investigator Responsibilities and Commitments
By signing Form FDA 1572, researchers confirm their dedication to a wide range of duties essential for the integrity and success of clinical studies. These duties encompass ensuring that the study adheres strictly to the approved protocol and complies with Good Clinical Practice (GCP) guidelines. Furthermore, researchers are responsible for meticulously documenting negative occurrences, an essential aspect in protecting participant safety and guaranteeing the dependability of study outcomes.
Maintaining accurate and comprehensive records forms another cornerstone of these responsibilities. This meticulous documentation is essential not only for regulatory compliance but also for the scientific validity of the research. Investigators must also facilitate informed consent processes, ensuring that participants are fully aware of the nature of the study, potential risks, and their rights. This process is integral to upholding the ethical standards outlined in the Declaration of Helsinki, which emphasizes the paramount importance of participant rights and safety.
Furthermore, investigators must prioritize the protection of participant data, aligning with the latest updates in GCP guidelines that underscore the necessity for robust data governance. This includes managing computer systems, safeguarding randomization processes, and ensuring the integrity of blinding procedures. These measures are essential in upholding the scientific rigor and ethical standards of research studies, ultimately aiding in the advancement of medical knowledge and the enhancement of patient outcomes.
Submission of Form FDA 1572
'The submission of Form FDA 1572 to the sponsoring organization or regulatory authority is a vital step that must be completed before the commencement of a research study.'. This requirement is integrated into the broader Investigational New Drug (IND) application process. Guaranteeing the prompt and precise submission of this form is vital to prevent regulatory delays, thus aiding a seamless transition into the practical stages of research. As stated by the FDA, which is tasked with protecting public health by regulating human and veterinary medications, vaccines, and other biological offerings, following these protocols is crucial for preserving the integrity and effectiveness of clinical studies. By following these guidelines, researchers can ensure that their studies progress without unnecessary interruptions, ultimately contributing to the advancement of medical knowledge and patient care.
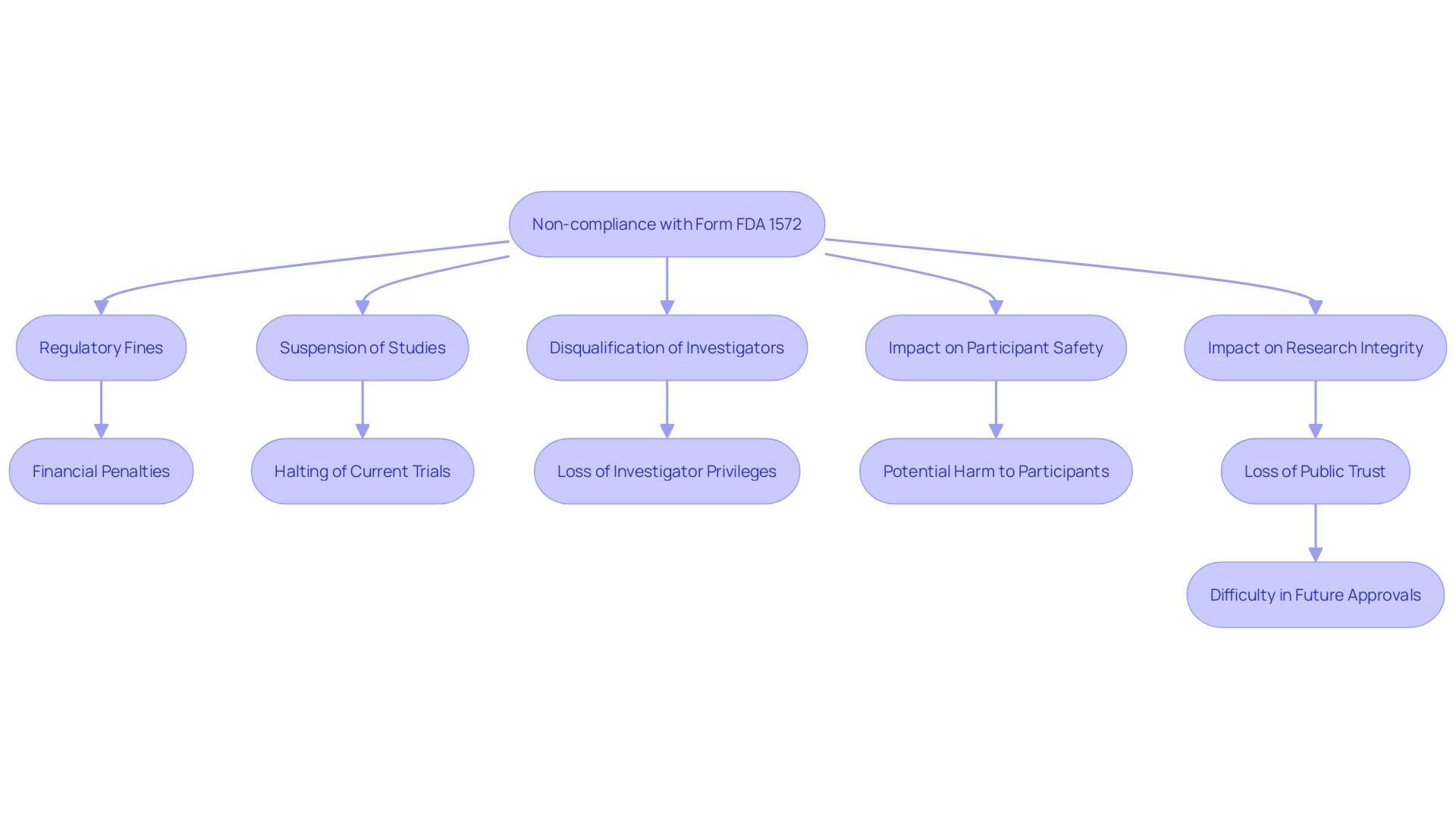
Consequences of Non-Compliance
Failure to comply with the requirements of Form FDA 1572 can lead to significant consequences for researchers and sponsors. Regulatory penalties for non-compliance can include fines, suspension of the study, or even disqualification of the investigator from future research. 'Non-compliance also poses a risk to participant safety and the integrity of research data, potentially compromising the entire research effort.'. According to the FDA, ensuring the safety and effectiveness of medical products is paramount, and adherence to regulatory standards is crucial to achieving these goals. Clinical trials, being a critical component of medical research, must be conducted with high and clearly defined standards to safeguard patient outcomes and maintain public trust in medical advancements.
Special Considerations for Foreign Clinical Studies
For clinical studies conducted outside the United States, Form FDA 1572 remains indispensable. Investigators in foreign locations are required to complete this form to comply with U.S. regulations, ensuring adherence to the same ethical and scientific standards mandated domestically. The FDA’s commitment to harmonizing human subject protection regulations underscores the importance of maintaining these standards globally. Special considerations must be taken into account, such as language barriers and differing regulatory frameworks. Translation quality is paramount, necessitating methods that ensure accuracy and purposefulness, beyond mere word-for-word translation. For instance, back translation is commonly used to evaluate translation quality, though it has its limitations. Additionally, understanding local regulatory environments is crucial. Investigators must meticulously ensure that all aspects of the form reflect local regulations while adhering to U.S. standards.
Conclusion
Form FDA 1572 serves as a foundational document in clinical research, ensuring that investigators are qualified and committed to maintaining regulatory standards throughout clinical trials. It not only delineates the responsibilities of the principal investigator and key personnel but also emphasizes the importance of protecting participant rights, safety, and welfare. By requiring comprehensive information about the study and the investigators involved, the form plays a critical role in regulatory oversight, ultimately safeguarding public health.
The completion of Form FDA 1572 is essential for all individuals involved in clinical trials, with particular emphasis on the principal investigator. This meticulous documentation is vital for maintaining accountability and ensuring compliance with regulatory expectations. The responsibilities outlined in the form, from strict adherence to protocols to the diligent reporting of adverse events, are integral to the integrity of clinical trials and the validity of the research findings.
Timely submission of Form FDA 1572 is paramount, as delays can hinder the progress of clinical trials and compromise public health initiatives. Non-compliance with the form's requirements can lead to severe consequences, including regulatory penalties that impact both investigators and participants. This highlights the necessity for thorough understanding and adherence to the outlined responsibilities.
For international studies, the form remains equally significant, ensuring that ethical and scientific standards are upheld across diverse regulatory environments. This global perspective is essential in maintaining consistency and integrity in clinical research, reaffirming the commitment to participant safety and effective medical advancements.




