Introduction
Contract Research Organizations (CROs) play a crucial role in advancing medical research, particularly in the biotechnology, pharmaceutical, and medical device sectors. These entities specialize in navigating the complexities of clinical trials, ensuring the safety and efficacy of new medical innovations.
One key player in this field is the CRO consultant, who brings a diverse set of skills to the table, ranging from data science to the interpretation of complex datasets. In this article, we will explore the role of a CRO consultant, the benefits of CROs in medical research, successful case studies, challenges faced by CRO consultants, and best practices for CRO consultants in the field of medical research. Join us as we delve into the world of CROs and their impact on advancing healthcare.
The Role of a CRO Consultant
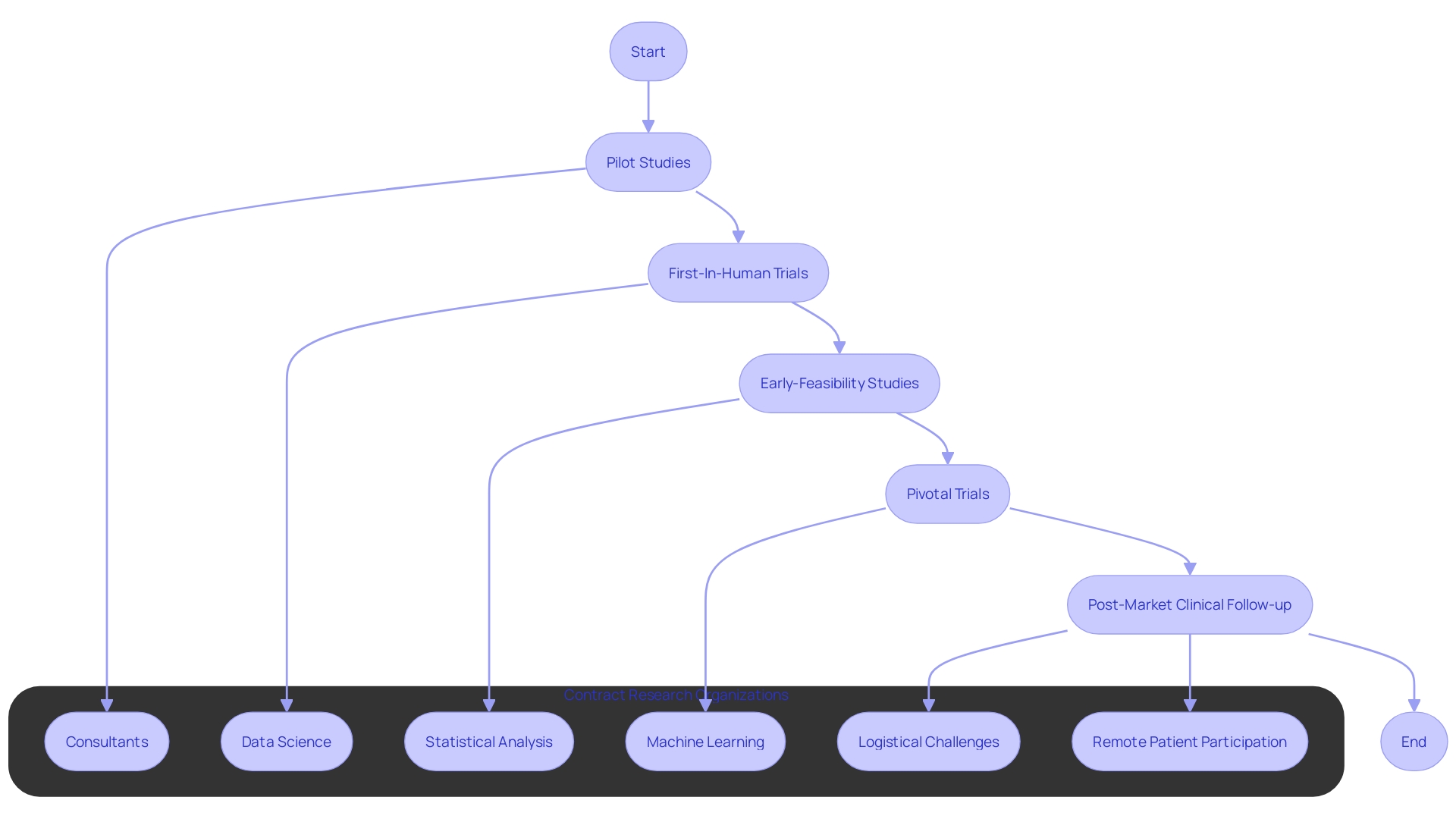
Contract Research Organizations (CROs) are integral to the progression of medical research, particularly for the biotechnology, pharmaceutical, and medical device sectors. These entities are adept at navigating the intricacies of clinical trials, which are essential for confirming the safety and efficacy of new medical innovations. A CRO consultant brings to the table a diverse set of skills, from data science to the interpretation of complex datasets, employing statistical methods and machine learning to extract meaningful insights.
This proficiency is indispensable for thorough analysis, ranging from market sizing to competitive evaluations and economic viability studies of medical interventions. Furthermore, CRO consultants offer critical evidence-based evaluations of current scientific developments, ensuring stakeholders make well-informed decisions. Their expertise is also vital in addressing logistical challenges, such as enabling patient participation in clinical trials from remote locations, overcoming obstacles related to travel and language differences.
At bioaccess™, our clinical research team, with over two decades of specialized experience in medtech, excels in providing high-quality, cost-effective CRO services in Latin America. We specialize in pilot studies, first-in-human (FIH), early-feasibility (EFS), pivotal, and post-market clinical follow-up (PMCF) studies. Our customized approach and deep understanding of the clinical trial landscape ensure that medical devices move through the trial phases efficiently, enhancing patient access to innovative treatments.
Benefits of CRO in Medical Research
Harnessing the transformative capabilities of artificial intelligence (AI) and machine learning (ML) in the healthcare sphere, Contract Research Organizations (CROs) are at the forefront of revolutionizing clinical trials. The integration of AI and ML not only enhances the precision and personalization of patient treatments but also elevates the efficiency and velocity of clinical study processes.
As health data burgeons, the vision for autonomous clinical trials is coming into focus, promising to overhaul the pharmacological landscape, optimize medical organization operations, and substantially improve patient outcomes. The advent of digital workflows and data management systems is a testament to how CROs are leveraging technology to ensure trials are not only more efficient but also adhere to the highest standards of data integrity and regulatory compliance. This digital transformation paves the way for a future where clinical trials are driven by data, analytics, and insights, ensuring that every study is conducted with unparalleled accuracy and ethical consideration.
Case Study: Successful CRO Implementation in Medical Research
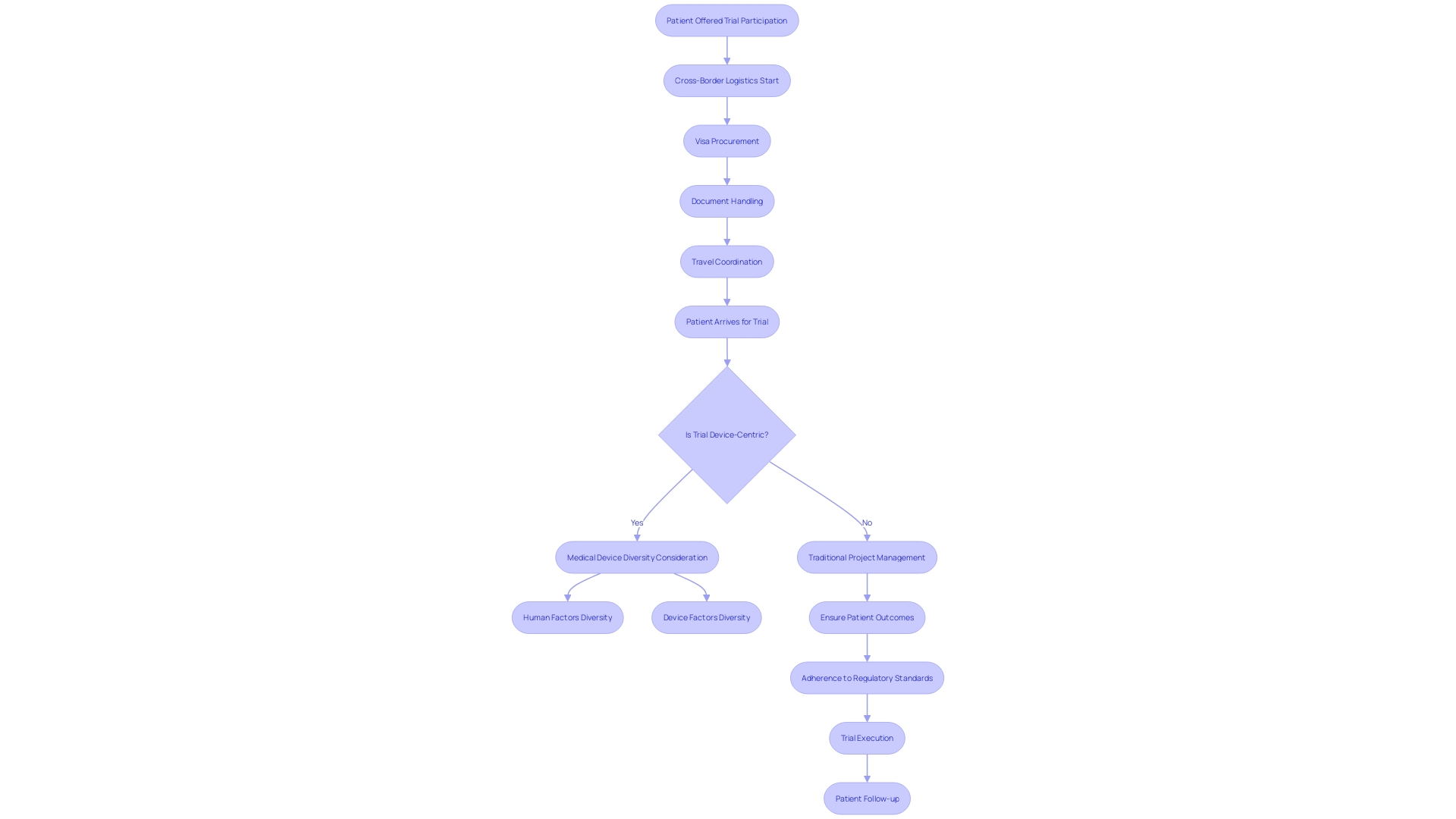
Clinical research organizations (CROs) are often at the forefront of bridging the gap between medical innovation and patient accessibility, particularly in cases involving ultra-rare diseases. For instance, consider the logistical and bureaucratic challenges faced by a patient in rural Pennsylvania afflicted with an ultra-rare disease.
When offered the chance to join a clinical trial for a potentially lifesaving treatment, the patient must navigate the complexities of international travel to Turkey, where the trial is conducted. This scenario involves intricate planning around visas, language barriers in document management, and the coordination of both air and ground travel. Such cases underscore the indispensable role of CROs in not only designing and managing clinical trials but also in facilitating patient participation, ensuring that cutting-edge treatments can reach those in dire need, regardless of geographic and logistical hurdles.
Challenges Faced by CRO Consultants in Medical Research
CRO consultants play a pivotal role in the progression of medical research, yet they are often confronted with intricate challenges. Consider the complexity of a situation where a patient from rural Pennsylvania, afflicted by an ultra-rare disease with no approved treatments, is presented with the chance to join a clinical trial in Turkey. This scenario underscores the multifaceted responsibilities of CRO consultants.
They must facilitate the patient's participation by addressing a myriad of logistical concerns, ranging from securing travel visas to managing essential documentation in a foreign language. Moreover, CRO consultants are tasked with the crucial duty of ensuring seamless communication between diverse parties, including sponsors, investigators, and regulatory bodies. Their ability to adeptly manage the evolving landscape of clinical trial regulations and swiftly adapt to each study's unique demands is a testament to their indispensable problem-solving acumen in the field of medical research.
Best Practices for CRO Consultants in Medical Research
Clinical Research Organization (CRO) consultants play a vital role in bridging the gap between medical innovations and patient accessibility. In the challenging scenario where a rural Pennsylvania patient with an ultra-rare disease is offered a chance to join a clinical trial in Turkey, the complexity of managing cross-border logistics services.
This highlights the necessity for CRO consultants to possess robust project management skills, ensuring that patients can navigate international trials with clear guidance on visa procurement, document handling, and travel coordination. Effective CRO consultants must integrate traditional project management expertise with the unique demands of the medical device industry, which prioritizes patient outcomes and adherence to regulatory standards.
The focus on patient safety and product quality is non-negotiable, as the ultimate goal is to enhance patient health through medical advancements. Moreover, understanding the user's perspective is indispensable, as it significantly influences project decisions. The intricate process of clinical trials, from the initial safety assessments in phase one involving healthy volunteers to the subsequent stages where efficacy and safety are evaluated in larger groups, including patients with the target condition, illustrates the depth of responsibility held by CRO consultants. Their commitment to ethical conduct and patient safety is paramount in navigating the multifaceted landscape of medical research.
Conclusion
In conclusion, CRO consultants play a vital role in advancing medical research by navigating complex clinical trials, providing critical evaluations, and ensuring the safety and efficacy of new medical innovations. The integration of artificial intelligence and machine learning enhances precision and efficiency in studies, while digital workflows ensure data integrity and regulatory compliance.
Successful case studies highlight the indispensable role of CROs in facilitating patient participation, even in cases with logistical challenges or ultra-rare diseases. They bridge the gap between medical innovation and patient accessibility, ensuring cutting-edge treatments reach those in need.
However, CRO consultants face challenges such as managing cross-border logistics, communication between diverse parties, and staying updated with evolving regulations. Their problem-solving acumen is crucial in navigating these complexities.
To be effective, CRO consultants must possess robust project management skills and integrate traditional expertise with the unique demands of the medical device industry. Prioritizing patient safety, product quality, ethical conduct, and understanding user perspectives are key to success. In summary, CRO consultants are instrumental in advancing healthcare by ensuring the safety and efficacy of new medical innovations through their expertise in data analysis, project management, logistical coordination, and adherence to regulatory standards. Their contributions drive progress in medical research while prioritizing patient outcomes and accessibility to innovative treatments.




