Introduction
Article Introduction:
Navigating the complex landscape of cross-border clinical trials can be a daunting task for patients and medical stakeholders alike. This is where CRO consultants come in.
In this article, we will explore the role of a CRO consultant, their key responsibilities, and the benefits of hiring one for medical research. From addressing logistical and linguistic barriers to integrating AI and ML technologies, CRO consultants play a pivotal role in revolutionizing the pharmaceutical industry and improving patient accessibility to critical medical interventions. Join us on this journey as we delve into the world of CRO consultancy and its impact on clinical trials.
What is a CRO Consultant?
A CRO consultant embodies the linchpin in navigating the complexities faced by patients and medical stakeholders in cross-border clinical trials. In a poignant scenario, they would guide a Pennsylvania patient suffering from an ultra-rare disease towards participating in a trial in Turkey, a daunting endeavor fraught with logistical and linguistic barriers. This level of consultancy is crucial, addressing queries on visa procurement, documentation translation, and travel arrangements—an intricate blend of regulatory savvy, cultural acumen, and personal care.
As healthcare technology advances, the role of the CRO consultant becomes increasingly pivotal. The potential for AI and ML to establish self-driving trials—digitally orchestrated and data-driven—can lead to highly personalized treatments and more efficient trials. CRO consultants are at the forefront of integrating these technologies to transform trial processes, promising unmatched accuracy in outcomes.
CMIC Group's pioneering effort, with over three decades as Japan's premier Contract Research Organization, showcases the expansive services offered by CROs. They epitomize end-to-end solutions tailored to clients' precise stages of drug development, from development and manufacturing to market entry strategies. This evolution from one-dimensional service to holistic support encapsulates the depth and breadth of expertise a CRO consultant brings to the table, driving the entire pharmaceutical value-chain, and revolutionizing patient accessibility to critical medical interventions.
Key Responsibilities of a CRO Consultant
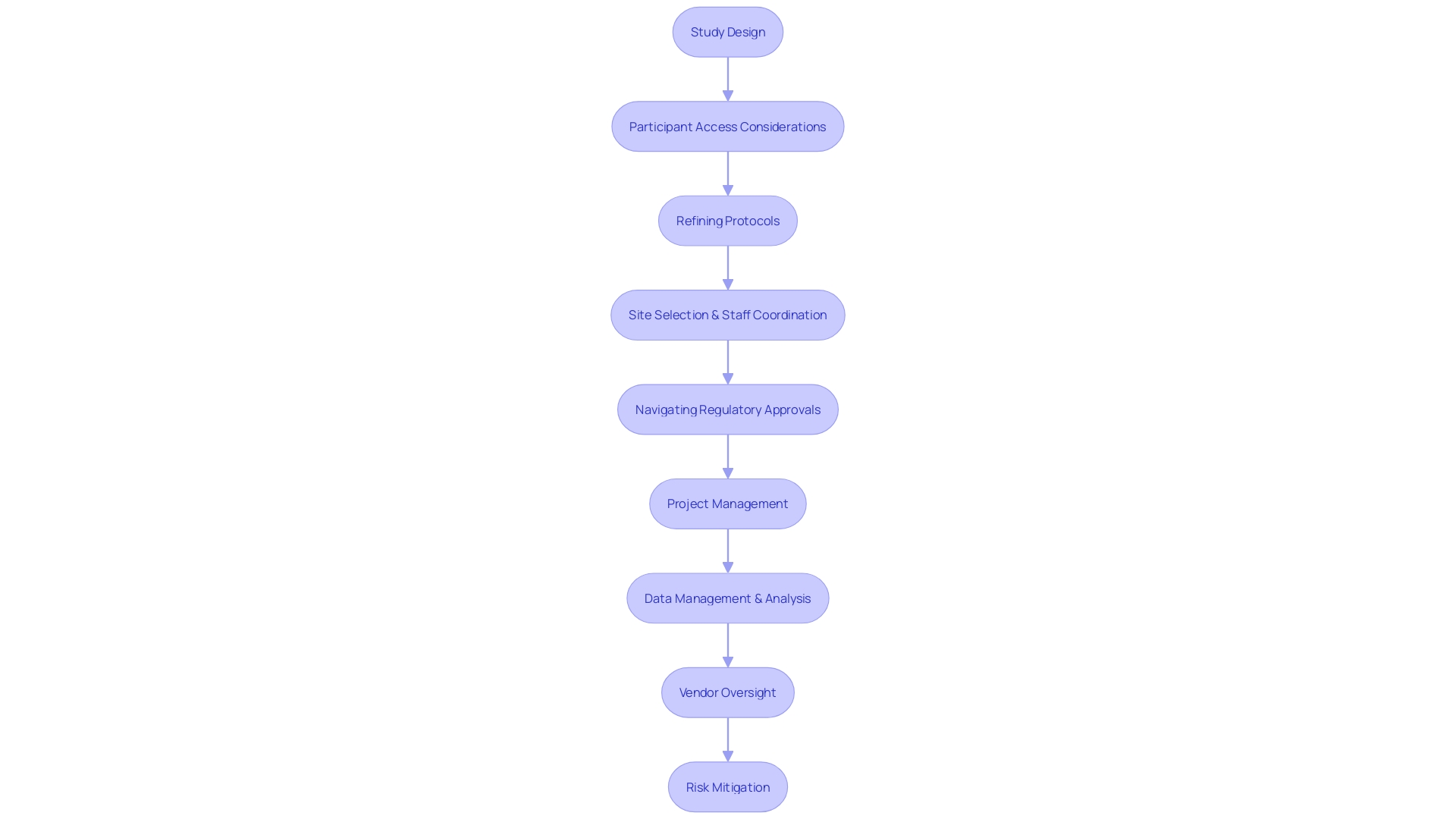
Clinical Research Organization (CRO) consultants must balance intricate scientific objectives with the logistical complexities of executing clinical studies. A key aspect of their role is to craft study designs that not only resonate with the research purposes but also consider participant access challenges, such as a patient from rural Pennsylvania having to navigate international travel to Turkey for a clinical trial. The role also involves refining protocols to be both scientifically rigorous and ethical, factoring in crucial considerations like the language barriers or document handling that international participants might face.
In managing site selection and relationships with staff, CRO consultants coordinate training and support to enhance the smooth progression of the trial, mirroring the industry's shift towards more efficient and effective operations. Ken Getz remarks on this evolution, highlighting a 'growing recognition in the importance of balancing great science with great execution' and leveraging technologies to improve support. To ensure studies adhere to stringent regulations, CRO consultants navigate the approval processes of different regulatory bodies, imperative for multi-regional trials with varied guidelines.
They are adept project managers, overseeing timelines and resource allocation, cognizant that decisions often made years in advance can greatly impact study outcomes. In data management and analysis, they partner with specialized teams to guarantee data integrity and insightful analysis, underpinned by robust quality control measures. The consultant's oversight of vendors extends to ensuring they deliver services up to par with the trial's needs.
Finally, addressing the potential risks inherent in clinical trials, the consultants must preemptively strategize to mitigate them. AP from Tree hill mentions the potential improvements in decision-making through rigorous planning, reflecting an industry trend towards optimizing each 'link in the chain' from an operational standpoint. CRO consultants thus embody the crucial intersection of scientific excellence and operational expertise required for the successful execution of clinical trials.
Benefits of Hiring a CRO Consultant for Medical Research
Partnering with a Contract Research Organization (CRO) consultant provides a robust framework for advancing medical research, capitalizing on a wealth of industry expertise to navigate complex clinical trials. The advisory role begins with the fundamental step of crystallizing a research question that guides the entire project scope. CRO consultants are uniquely equipped to design studies that minimize confounding factors, leveraging their deep understanding of dependent and independent variables and the critical function of control groups in yielding meaningful data.
Amidst a landscape where patient access to trials presents logistical challenges, such as the daunting prospect of a Pennsylvania patient needing to travel to Turkey for a trial, the CRO's role becomes even more pivotal. They harness resources efficiently, ensuring judicious use of budgets and optimizing the allocation of personnel and equipment. Access to the consultant's network allows for specialized skills to be channeled into pivotal areas of the trial, such as statistical analysis, by emphasizing the use of AI and machine learning technologies that are revolutionizing data management and patient treatment personalization.
Crucially, CRO consultants expedite regulatory navigation, which facilitates quicker study start-ups. Their acumen for quality assurance activities ensures superior compliance and data integrity. By preemptively identifying risks, consultants strategically implement mitigation plans to minimize setbacks, enhancing the overall success rate of clinical trials.
An overarching advantage of engaging CRO consultants is their capacity to enable research organizations to concentrate on their core competencies. This recalibration of focus is vital, as backed by industry insights highlighting that early-stage decisions, if more meticulously planned, can profoundly impact the outcome of studies. By entrusting operational management to these consultants, researchers can devote their energies to the innovation and advancement of medical science, knowing the logistical and administrative burdens are expertly shouldered by their partners in clinical research.
Conclusion
In conclusion, CRO consultants are essential in cross-border clinical trials to overcome logistical and linguistic barriers, integrate AI and ML technologies, and improve patient access to critical medical interventions. Their responsibilities include designing rigorous studies, navigating regulations, and mitigating potential risks. Partnering with CRO consultants provides a framework for advancing medical research by optimizing study designs, resource allocation, and regulatory compliance.
They expedite study start-ups, ensure data integrity, and allow research organizations to focus on core competencies and innovation. Overall, CRO consultants play a pivotal role in revolutionizing clinical trials, enhancing efficiency, accuracy, and patient accessibility. Their expertise drives advancements in medical research, ultimately improving patient outcomes and saving lives.




