Introduction
Active control trials are a fundamental component of medical research, providing valuable insights into the efficacy and safety of new treatments. These trials adhere to strict ethical standards, ensuring participant welfare through informed consent and rigorous monitoring. However, the landscape of active control trials is evolving, with the integration of innovative methods like platform trials and the need for human oversight in critical decision-making areas.
Despite their importance, active control trials have limitations, such as potential biases in selecting active controls and the influence of confounding factors. Interpreting the results of these trials poses unique challenges, requiring an understanding of clinical significance, study design, and data quality. Noninferiority and equivalence testing play crucial roles in comparative analysis, but their success hinges on meticulous planning and transparent communication.
Real-world case studies demonstrate the practical applications of active control trials in various medical domains, highlighting their impact on patient care and the advancement of medical science. Overall, active control trials serve as a cornerstone in clinical research, shaping the trajectory of medical practice while addressing ethical, social, and regulatory dimensions.
Ethical Considerations in Active Control Trials
Active control experiments, pivotal in medical research, compare new treatments against established standards or placebos to assess their efficacy and safety. These experiments are guided by strict ethical standards to safeguard participant welfare. Informed consent is crucial, ensuring participants are fully aware of the study's scope and potential risks before participation. Participant safety is closely monitored, often involving Data Monitoring Committees (DMC) which provide oversight to maintain the integrity of the study, as per the FDA's draft guidance FDA-2001-D-0219. Confidentiality is strictly upheld to protect sensitive participant data.
The moral environment of such experiments is developing with the incorporation of inventive approaches like platform studies, which assess multiple interventions more effectively, as discussed in 'Design of platform studies with a change in the control intervention arm' by Peter Greenstreet et al. These trials adjust as new standards of care emerge, ensuring ongoing therapies are compared against the most current and effective options. While Equal Randomisation (ER) is a common method for assigning interventions due to its simplicity, it's important to note that it does not maximize statistical power, contrary to popular belief. Alternative methods like Thompson Sampling (TS), which aligns the probability of assignment to treatment with its likelihood of being the best option, can offer greater power.
Within the broader scope of ethical considerations, the Declaration of Helsinki stands as a cornerstone document for medical research ethics. Yet, it faces scrutiny and calls for revision to better align with contemporary research practices. As artificial intelligence gains prominence in healthcare, its role in ethical decision-making processes is also under examination, invoking discussions on human oversight in critical decision areas. These discussions are mirrored in the realm of autonomous weapons systems, where global leaders and ethical bodies advocate for strict regulations and human oversight of AI decision-making. These discussions emphasize the crucial requirement for human supervision and ethical management in all domains involving life-changing decisions, including the implementation of experiments with a comparative group.
Limitations of Active Control Trials
Active comparison groups play a critical role in clinical trials, offering comparative insights that help establish the efficacy of new treatments. Nevertheless, the choice of a participating influence is a procedure filled with potential prejudices. It is crucial to select a comparative intervention that mirrors the existing standard of care to produce outcomes that are dependable and relevant to actual medical practice. The engaged management must be carefully selected to avoid biased data, which could endanger the experiment's legitimacy.
Moreover, confounding factors are another significant concern in active control trials. These are variables that can influence the outcome of the study unintentionally. Researchers must meticulously design studies to mitigate the risk of confounding factors. This involves robust statistical methods, like Equal Randomisation (ER) and Thompson Sampling (TS), to ensure a fair and unbiased allocation of interventions to participants. In an experimental design, where interventions are assigned randomly in a 1:1 ratio, is straightforward but may not always optimize statistical power. On the other hand, TS adapts the probability of assignment based on ongoing results, which may offer a more refined approach to treatment allocation.
Recent advancements in clinical research design underscore the importance of addressing these challenges. Peer-reviewed journals emphasize the necessity of innovative research and the implementation of fresh ideas to advance the field. As an instance, a meta-analysis of cardiology studies emphasized the potential advantages of IV-magnesium in post-myocardial infarction care, demonstrating the influence of well-executed comparison studies. As such, the clinical research community is continuously seeking ways to improve research designs to enhance patient care outcomes and healthcare delivery overall.
Interpreting Active Control Trials: Challenges and Solutions
In the complex terrain of clinical trials, deciphering the outcomes from comparison groups presents distinctive difficulties. The core issue lies in determining the actual clinical impact of the results. It's not merely about establishing statistical significance but understanding the real-world implications for patient care. For example, researchers must determine whether observed distinctions between a new approach and the engaged intervention are medically significant. The task is to ensure that any claims of a new treatment being superior or noninferior are backed by evidence that extends beyond mere numbers.
The study design is a pivotal element that influences interpretation, as it frames the context within which results are evaluated. Sample size also plays a crucial role; too small, and the findings may not be generalizable; too large, and the risk of detecting meaningless differences increases. Furthermore, the quality of the data collected is paramount, as it forms the backbone of reliable and valid results.
Insights from a study published in BMJ Global Health shed light on the complexities of active control experiments. Researchers, including associate professor Alex Perkins from Notre Dame, utilized mathematical modeling to unravel biases in dengue virus transmission studies in Indonesia. They emphasized that controlling for transmission coupling between humans and mosquitoes is a challenge that traditional clinical analysis does not typically address. This points to the need for innovative methodologies to mitigate biases not just in design but also in the interpretation phase.
Furthermore, the development of clinical examination structure, particularly in the field of cardiology, highlights the significance of adjusting to new proof. Adaptive designs, as developed by CDER statisticians for bioequivalence studies, underscore the necessity to evolve methodologies to reflect the variable nature of drugs and diseases. This is crucial in accurately interpreting the results of active control experiments and in making informed decisions for patient care.
As Frank David, a clinical experiments expert, emphasizes in his introduction to the updated Stat's Guide to Interpreting Clinical Experiment Results, the tools and advice for digesting experiment data are indispensable in today's landscape. The guide provides a framework for navigating the complexities of examination outcomes, ensuring that consumers and practitioners alike can make sense of the data.
In brief, the interpretation of studies involving intervention comparison is a complex procedure that necessitates a comprehensive comprehension of the clinical importance, study structure, sample magnitude, and data reliability. With the right approach, bolstered by advanced methodologies and expert guidance, researchers can navigate these challenges and contribute to the advancement of medical science and patient care.
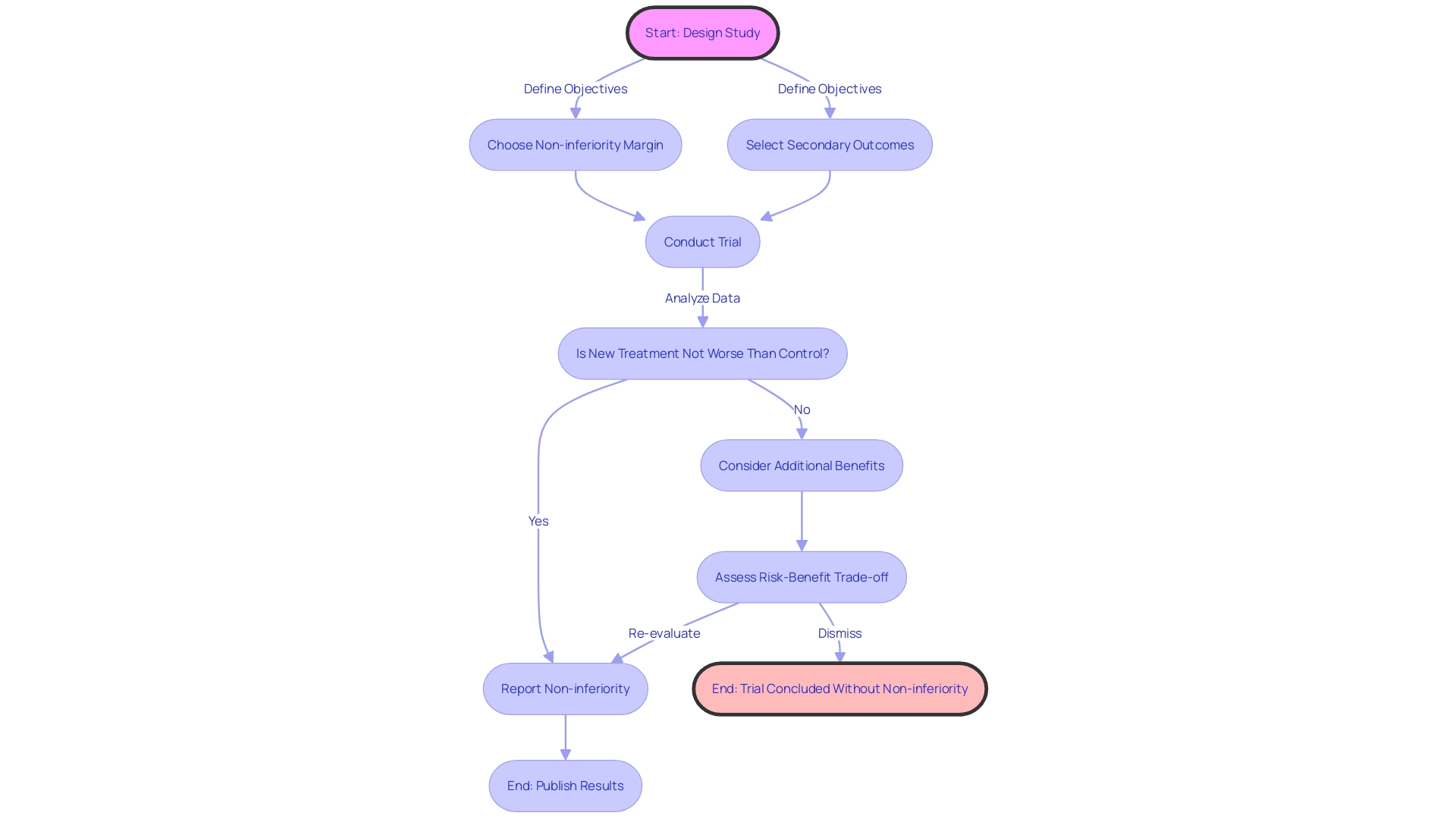
Noninferiority and Equivalence Testing in Active Control Trials
In the realm of trials involving management, noninferiority and equivalence testing serve as cornerstones for comparative analysis. Noninferiority experiments are created to demonstrate that the effectiveness of a new therapy is not considerably lower than that of a current standard by a pre-established threshold. On the other hand, equivalence studies aim to show that the effectiveness of a new intervention is within an acceptable range when compared to the active comparator. The complexities of these experiments require careful planning in relation to study protocols, participant numbers, and statistical methodologies to verify that outcomes are both reliable and meaningful.
Examining more closely, platform experiments embody a creative method for assessing multiple therapies simultaneously, as opposed to conducting separate studies for each novel therapy. This framework is especially important when a new therapy replaces the reference as the standard of practice, after which it serves as the yardstick for evaluating other interventions. These experiments emphasize the significance of deciding whether to keep or disregard previous information when the managing procedure is modified. Research by Peter Greenstreet et al. has shown that preserving data prior to updating the control can inadvertently reduce the study's power to detect meaningful differences.
Additionally, the objectives of noninferiority studies can go beyond simple efficacy comparisons. For example, a new approach that provides significant benefits—such as decreased side effects or shorter durations of therapy—may be taken into account despite efficacy outcomes that are just shy of meeting noninferiority criteria. This highlights the need to clearly define study objectives and to take into account the wider risk-benefit profile of new treatments.
In light of these considerations, the FDA has underscored the need for clarity and accessibility in communicating information, particularly regarding the major side effects and contraindications of drugs in direct-to-consumer advertising. This principle of clear and conspicuous presentation of critical information parallels the need for clarity in the design and analysis of clinical studies, ensuring that the results are comprehensible not just to clinicians, but also to the wider public who might be affected by these outcomes.
Ultimately, the success of noninferiority and equivalence studies depends on the ability to make informed decisions about the objective of the investigation, which in turn guides the selection of appropriate non inferiority margins and secondary outcomes, thereby influencing the analysis and reporting of study results. Such methodological rigor is essential for advancing clinical practice and enhancing patient care.
Case Studies: Real-world Applications of Active Control Trials
Active control experiments are a foundation in the field of clinical research, offering essential comparative data that can expedite the advancement of new therapies and enhance patient management across different medical domains. A tangible illustration of this can be seen in the field of HIV prevention, where the design of active-controlled experiments has been pivotal. According to a paper titled 'Active-Controlled Trial Design for HIV Prevention Trials,' such studies enable the evaluation of new interventions against the standard of care, rather than a placebo, ensuring ethical integrity when known effective treatments exist.
In surgical practices, incorporating experiments with dynamic management into regular procedures can improve the quality of treatment. An example of this is in radical prostatectomy, where researchers randomize certain aspects of the surgery to refine techniques and improve patient outcomes. This approach not only enriches clinical practice but also embeds research into daily medical procedures, allowing for continuous improvement.
The incorporation of engaged management groups in experiments likewise tackles vital lawful, moral, and public concerns that arise in the advancement of up and coming advancements. By leveraging real-world data and implementing complex interactive designs, such as Bayesian methodology, researchers can navigate the evolving regulatory landscape with greater precision. This adaptability is reflected in draft guidance from the FDA that emphasizes the selective use of historical patient-level information over summary estimates, as highlighted by industry commentators.
Furthermore, the utilization of engaged monitoring groups is not just a methodological decision but also a tactical one, impacting the market worth of research. With the clinical experiment market estimated to grow significantly, the incorporation of active control studies could contribute to this industry expansion.
In the end, the adoption of real-world data to inform experiment design and execution has been encouraged by health authorities. For example, the FDA's recent guidance on clinical experiment diversity highlights the significance of such data in ensuring that experiment populations accurately represent the real-world patients who will ultimately use the drugs. The notion of 'fit for purpose' data is gaining traction, whereby the quality of real-world data is defined by its relevance to specific research questions and its reliability in informing scientific inferences.
In summary, active control trials not only fulfill a scientific role but also address a spectrum of ethical, social, and regulatory dimensions, thereby shaping the trajectory of clinical research and patient care. These real-world applications serve as a blueprint for future endeavors, ultimately aiming to enhance patient outcomes and advance medical practice.
Conclusion
Active control trials are a fundamental component of medical research, providing valuable insights into the efficacy and safety of new treatments. These trials adhere to strict ethical standards, ensuring participant welfare through informed consent and rigorous monitoring. The ethical landscape of active control trials is evolving with the integration of innovative methods like platform trials and the need for human oversight in critical decision-making areas.
Despite their importance, active control trials have limitations, such as potential biases in selecting active controls and the influence of confounding factors. Interpreting the results of these trials poses unique challenges, requiring an understanding of clinical significance, study design, and data quality. Noninferiority and equivalence testing play crucial roles in comparative analysis, but their success hinges on meticulous planning and transparent communication.
Real-world case studies demonstrate the practical applications of active control trials in various medical domains, highlighting their impact on patient care and the advancement of medical science. These trials have played a pivotal role in fields such as HIV prevention and surgical practices, enhancing the standard of care and embedding research into daily medical procedures. The integration of active control trials also addresses legal, ethical, and social considerations in the development of emerging technologies.
In conclusion, active control trials serve as a cornerstone in clinical research, shaping the trajectory of medical practice while addressing ethical, social, and regulatory dimensions. The interpretation of these trials requires careful consideration of clinical significance, study design, sample size, and data quality. By navigating these challenges and leveraging innovative methodologies, researchers can contribute to the advancement of medical science and patient care.




