Introduction
Active-controlled trials are a cornerstone of medical research, providing a comparison between new treatments and standard ones. These trials play a crucial role in determining the efficacy and safety of new interventions by comparing them against existing effective treatments rather than a placebo. However, navigating the challenges in interpreting active-controlled trials is essential to ensure reliable results.
This article explores the selection of the active control group, patient adherence, treatment response variability, and potential biases. It also delves into the ethical considerations, the comparison with placebo-controlled trials, non-inferiority trials, alternative metrics, and the applications and limitations of active-controlled trials. By understanding these complexities, researchers can conduct meticulous active-controlled trials that advance medical knowledge and contribute to evidence-based decision-making.
Challenges in Interpreting Active-Control Trials
Active-controlled trials are a cornerstone of medical research, providing a comparison between new interventions and standard ones, known as the active control group. The selection of this group is crucial, as it must accurately represent the standard approach in the population studied. To ensure reliability, researchers must navigate patient adherence, response variability, and potential biases. For example, a document titled 'Contemporary methods for assessing variability in the impact of interventions from experiments and real-world data' illuminates the complexity of these experiments. Variability in the effects of medical interventions, as Sir William Osler observed in 1892, is a long-established acknowledgement that individuals react differently to interventions, emphasizing the need for customized clinical investigations. The coming together of increasing negative or marginal effect experiments and the desire for understanding differential treatment effects for subgroups reinforces the importance of meticulous active control group selection. Furthermore, adaptive study designs have been suggested for assessing bioequivalence in highly variable drugs, demonstrating the developing approaches to address these challenges. The revision of a major 2020 report on experiments emphasizes the continuous significance of such insights, with experiments specialist and physician Frank David emphasizing the 'lessons and concise guidance' as 'more valuable than ever today.' With the constant progress of the medical field, the principles of active-controlled studies continue to be a dynamic and crucial aspect of clinical research.
Comparison with Placebo-Controlled Trials
Active-controlled experiments play a crucial role in the assessment of novel therapies, providing a comparative perspective in relation to established interventions. These experiments are important because they don't just assess effectiveness in isolation, but compared to a recognized active remedy, which is priceless in comprehending the relative efficiency of new measures. Such comparisons can elucidate whether a new approach outperforms, is on par with, or falls short of existing standards, thus equipping healthcare professionals with crucial data for evidence-based decision-making.
The ethical and practical dimensions of active-controlled experiments are manifold, as they must navigate a complex landscape of market forces, intellectual property rights, and societal goals. They're structured to tackle key questions about the international state of technology, legal frameworks, and the broader implications of research on human well-being. This is exemplified by the historical evolution of Hematopoietic Stem Cell Transplantation (HSCT), which was initially reserved for terminally ill patients but has since expanded its ethical considerations alongside technological advancements.
Recent news highlights the significance of such research, with Menarini Group's ongoing studies on Elacestrant in metastatic breast cancer and the comprehensive analysis by Lankenau Institute for Medical Research (LIMR) on the use of paclitaxel-coated devices for Peripheral Arterial Disease (PAD). These examples underscore the continuous effort to enhance patient outcomes through innovative therapeutic approaches.
In the realm of non-inferiority investigations, the aim is to demonstrate that a new therapeutic approach is at least not significantly worse than the standard, while possibly offering other advantages, such as fewer side effects or shorter duration of intervention. These trials are essential in the context of diseases where established interventions exist, and the enhancement of patient care is sought through incremental but meaningful advances in therapeutic options.
The U.S. Food and Drug Administration's Center for Drug Evaluation and Research (CDER) plays an instrumental role in the approval of new treatment options, ensuring that these meet the necessary criteria for safety and efficacy. Every year, CDER evaluates a range of new molecular entities and therapeutic biological products, some of which are pioneering in practice, representing the forefront of medical progress.
To summarize, active-controlled experiments are not just a methodological decision but a strategic method to medical research that aligns with the changing ethical, legal, and social environments of medical technology. They offer a strong structure for evaluating new therapies in an actual-world setting, thereby promoting informed medical decisions and ultimately progressing healthcare delivery.
Ethical Considerations in Active-Controlled Trials
Active control groups play a crucial role in trials, especially when it comes to ethical aspects. These groups must receive an established standard of care to ensure participant safety and effectiveness. The ethical selection of control groups is paramount to preclude any undue harm or biases. Adhering to ethical guidelines, researchers implement informed consent protocols, proper randomization, and blinding techniques to safeguard the ethical standards of the study and protect participant rights. Recent discussions in the literature, such as those found in the paper 'Design of platform experiments with a change in the control intervention arm,' underscore the importance of thoughtful experimental design that aligns with ethical considerations and the dynamic nature of standard care within medical research.
Non-Inferiority Trials and Effect Preservation
Active-controlled non-inferiority studies are crucial in clinical research when a placebo-controlled design is not feasible or ethical. These assessments evaluate whether a novel therapeutic approach's effectiveness is not significantly inferior to that of a well-established active intervention, taking into account a predetermined non-inferiority threshold. The intent is not only to evaluate efficacy but also to discern additional benefits of the new treatment, such as reduced side effects or shorter treatment duration, which may be particularly relevant for patient care. Rigorous statistical methods are applied to ensure that the non-inferiority of the new intervention is reliably demonstrated. This involves a careful selection of the non-inferiority margin based on both efficacy and potential benefits, which should then guide the study's design, execution, and interpretation of results. Transparent reporting and a clear articulation of the experiment's objectives, including the risk-benefit assessment, are essential for translating findings into clinical practice and informing healthcare decisions.
Alternative Metrics: The Averted Events Ratio
In the realm of active-controlled trials, the averted events ratio emerges as a pivotal metric, offering a transparent comparison between a novel intervention and the established active control. By quantifying the reduction of adverse events achievable with the new approach in contrast to the control, this metric illuminates the tangible benefits for patient outcomes. It is an essential instrument for researchers and clinicians to determine the effectiveness and safety profile of emerging therapies. A recent study, encapsulated in the paper titled 'Survival analysis for Adverse events with VarYing follow-up times (SAVVY),' extends the conventional approaches, offering a comprehensive overview of findings that could reshape future safety analyses in medical experiments. The SAVVY study presents a roadmap for integrating innovative approaches to evaluating impact on medical care, underscoring the commitment to enhancing medical knowledge and clinical practices.
Applications and Limitations of Active-Controlled Trials
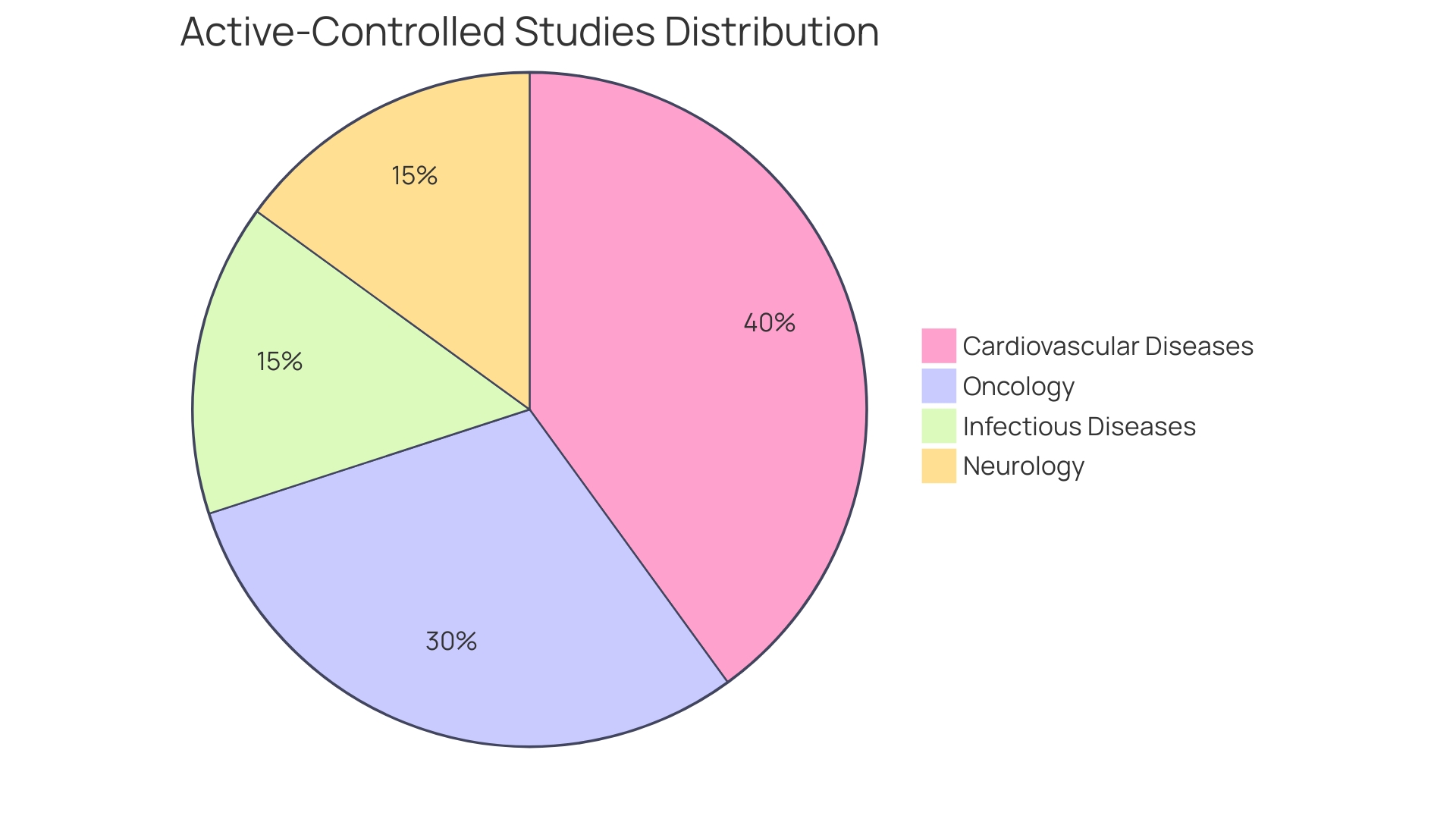
Active-controlled studies are crucial in advancing medical research, especially in fields like cardiovascular diseases, oncology, infectious diseases, and neurology. They play a critical role in determining the efficacy and safety of new interventions by comparing them against existing effective treatments rather than placebo. For example, the innovative R-RCT design, which integrates real-world registry data with randomized experiment features, was adopted in the DAPA-MI study to efficiently enroll a large patient cohort while maintaining the rigor of causal inference.
Despite their usefulness, active-controlled experiments are not without difficulties. Possible prejudices, the variety of patient populations, and the complexities involved in maintaining blinding protocols are all factors that could impact the integrity of experiment outcomes. This complexity was evident in the DAPA-MI study, where an unexpected reduction in the anticipated primary outcomes necessitated careful consideration of the study's conclusions.
The FDA's changing regulatory environment, including draft guidance on the use of historical data and complex interactive designs, highlights the significance of careful experiment design. For instance, the use of historical controls can be advantageous in rare disease research, where patient numbers are limited. The approval of Koselugo (selumetinib) for children with neurofibromatosis type 1, which relied on historical data, exemplifies this approach.
Moreover, engagement of patients and the general public in research on clinical trials is gaining traction, with activities that span from prioritizing research questions to sharing research findings. This partnership between researchers and the public ensures that experiments are relevant, well-conducted, and the outcomes are effectively communicated to those who need them.
Given the connection between Parkinson’s disease and a greater likelihood of autoimmune disorders, the importance of active-controlled investigations in revealing intricate disease interactions becomes even more notable. The observations from such experiments can advise medical practice and ultimately contribute to individualized patient care. Hence, when analyzing the results of active-controlled studies, it is crucial to take into account their restrictions and the wider framework of the clinical and regulatory setting.
Case Study Examples: Active-Controlled Trials in Different Disease Areas
Active-controlled studies are crucial in the evaluation of new interventions in different medical fields, and their structure must carefully consider ethical, legal, and global criteria to guarantee strong and socially advantageous results.
In the realm of cardiovascular disease, an active-controlled study examined a new anticoagulant aimed at reducing stroke risk in atrial fibrillation patients. This trial not only measured the drug's effectiveness against the standard therapy but did so while navigating the complex legal and regulatory landscape that governs such research, ensuring that the social goal of enhancing patient outcomes was achieved without compromising safety or ethical standards.
Oncology research embraced a similar approach when analyzing a novel targeted therapy for advanced lung cancer. The comparative effectiveness and safety of this intervention against an active control were discerned, with the overarching aim of improving overall survival rates. Scientific antecedents and ethical considerations were foundational to the study's design, reflecting international practices in the evolution of cancer treatments.
In the infectious disease sector, the effectiveness of a new antibiotic was assessed through an active-controlled study. Participants' clinical response rates and adverse events were meticulously recorded and compared with those of the active control group, all while adhering to a framework of ethical questions and cross-sectoral analysis that spanned academia, healthcare, and private sectors.
Neurological disorders, such as migraine headaches, also benefit from active-controlled experiments. A new drug's efficacy was measured by its ability to reduce the frequency and severity of migraines in comparison to an established treatment. This study exemplified the harmonious blend of real-world data and randomized experiment features, aiming for a comprehensive understanding of therapeutic impact within a well-defined ethical and legal context.
Each of these case studies highlights the crucial role active-controlled trials play in advancing medical science, with a strong emphasis on ethical considerations, international context, and the goal of enhancing human health while ensuring patient safety and adherence to regulations.
Conclusion
In conclusion, active-controlled trials are crucial in medical research, providing a comparison between new treatments and standard ones. The selection of the active control group is essential, and researchers must navigate challenges such as patient adherence, treatment response variability, and potential biases to ensure reliable results. Active-controlled trials offer advantages over placebo-controlled trials as they compare against established active treatments, aiding evidence-based decision-making.
Ethical considerations are paramount, with informed consent, randomization, and blinding techniques ensuring participant safety. Non-inferiority trials are vital when established treatments exist, aiming to show that a new treatment is not significantly worse than the standard. Alternative metrics like the averted events ratio provide transparent comparisons, quantifying the reduction of adverse events with a new treatment.
Active-controlled trials have applications in cardiovascular diseases, oncology, infectious diseases, and neurology, advancing medical research and personalized patient care. However, potential biases and the complexity of maintaining blinding protocols should be recognized. Meticulous trial design, adherence to ethical guidelines, and considering the clinical and regulatory environment are essential for reliable active-controlled trials.
By understanding and addressing these complexities, researchers can contribute to evidence-based decision-making and advance medical knowledge.




