Introduction
The field of clinical data management is undergoing a significant evolution, moving beyond its traditional role of ensuring data accuracy and consistency. Today, data management involves extracting actionable insights using advanced technologies and methodologies. Clinical Data Repositories (CDRs) play a crucial role in this transformation, providing real-time, patient-centered information that allows healthcare providers to make informed treatment decisions.
The integration of AI-powered algorithms and risk modeling further enhances this evolution, enabling medical professionals to access comprehensive patient medical histories and improving patient care. The adoption of Electronic Data Capture (EDC) systems and electronic Patient Reported Outcomes (ePRO) is also revolutionizing clinical data management, offering immediate data entry and enhancing data precision. These technologies, along with the integration of machine learning and big data analytics, are shaping the future of clinical data management, leading to improved patient outcomes and more personalized care.
Despite the challenges posed by managing large volumes of data, innovative data management strategies are being developed to meet the unique needs of healthcare entities. Ensuring data quality and compliance in clinical trials is crucial, as substandard data can lead to overlooked patterns and relationships. Looking ahead, the integration of machine learning, data warehousing, and data science will continue to transform the field, unlocking new possibilities for medical research and improving patient care.
The Evolution of Clinical Data Management: From Policing to Insight Extraction
The function of clinical information administration is experiencing a significant change. Historically, this area was associated with guaranteeing the precision and uniformity of information. Yet, today's landscape demands a more dynamic approach. Using cutting-edge technologies and methodologies, information management now involves extracting actionable insights that drive scientific research forward. For example, the utilization of Clinical Data Repositories (CDRs) has become integral. These databases provide real-time, patient-centered information that healthcare providers can access swiftly, facilitating better treatment decisions. Through CDRs, professionals can access a patient's comprehensive health history, including prior procedures and test results, which effectively reduces unnecessary testing and streamlines care.
The adoption of CDRs also opens the door for advanced risk modeling and prediction through AI-powered algorithms, which can extract clinical information from these repositories with ease. This progress is wonderfully demonstrated by the collaboration between healthcare providers and IT experts, like Harmony Healthcare IT, who handle outdated information with a careful focus on security and compliance with legal records. Health information management teams play a pivotal role in validating this data, ensuring accuracy and continuity. Moreover, platforms like PRISMA demonstrate how technology can address pressing needs by enabling access to fragmented medical histories, thereby enhancing patient care.
As AI and machine learning (ML) continue to integrate into healthcare systems, they bring the promise of reviewing notes related to patient care with near-human accuracy. This capability is not just theoretical; it's actively improving patient treatment options. For instance, AI has supported clinical trials for conditions like uveal melanoma with liver metastasis, which lacks FDA-approved treatments. Despite the cautious nature of the pharmaceutical industry regarding AI integration into regulated workflows, its potential to transform information management and patient outcomes is undeniable.
It's crucial to recognize that the road to fully integrating AI into healthcare is a work in progress. Clinicians still encounter difficulties in extracting pertinent information from the overwhelming amount of information, frequently exacerbated by the constraints of outdated EHR systems. Yet, strides are being made: companies like UHS are developing solutions that enhance the usability and safety of EHRs, focusing on nursing documentation, physician communication, and pharmacy workflows. The objective is evident—AI should enhance the influence of medical information, directing us towards a future where every bit of knowledge enhances patient care and treatment results.
Key Activities in Clinical Data Management
The handling and organization of clinical information plays a crucial role in the realm of medical research, providing the groundwork for accurate and reliable clinical facts which, in turn, propels healthcare advancements. The main responsibilities of clinical information handling encompass the precise procedures of collecting information, diligent practices for entering information, meticulous information cleansing, rigorous information validation, and comprehensive information analysis. Each function is intricately linked to preserving the information's integrity, ensuring that its accuracy stands up to scrutiny. For example, pediatric clinics such as Bright Future Pediatrics prioritize the trust and confidence of caregivers, making efficient handling of information crucial to uphold their reputation as a 'Parents' Choice Provider'. In the same way, the CCC19 initiative showcases the significance of openness and availability in medical information, providing tools such as explanatory videos and Q&A forums to clarify research discoveries for the general public. The effectiveness and dependability of handling information are further demonstrated by UHS's integration of solutions into the Oracle EHR system, enhancing user satisfaction and safety. These endeavors highlight the essential role of services related to the handling and analysis of medical information in promoting innovation and comprehension within the healthcare sector, ultimately resulting in enhanced patient results.
Advantages of Electronic Data Capture (EDC) and Electronic Patient Reported Outcomes (ePRO)
Electronic Data Capture (EDC) systems and electronic Patient Reported Outcomes (ePRO) are changing the landscape of clinical information management. EDC systems enable prompt information input, greatly reducing the likelihood of mistakes and enhancing information accuracy. Meanwhile, ePRO empowers patients to report their health outcomes directly, enhancing both the quality of information and patient involvement. The integration of these technologies by Contract Research Organizations (CROs) has propelled the efficiency and success of medical research to new heights.
In contrast to traditional paper-based methods, which are prone to issues like missing or ambiguous entries, EDC and ePRO provide a clear advantage. These technologies enable information to be captured as it occurs, ensuring adherence to the protocol and maintaining information integrity. Bill Byrom, an authority on clinical outcome assessment information collection, underscores the challenges of paper methods, including the practical difficulty of querying information riddled with inconsistencies. A study highlighted by Byrom involving the SF-36 questionnaire found that 44% of participants either omitted or ambiguously completed items, illustrating the scale of information quality issues encountered with paper-based collection.
Moreover, recent advancements in digital information collection tools, such as connected devices and wearables, alongside AI-driven analysis techniques, afford an unprecedented depth of insights. These insights are essential to patient-centered drug development and require a well-defined information strategy from the outset to handle the vast information volumes effectively. Such strategic planning is crucial to leverage information for informed decisions that prioritize patient safety and data integrity.
The adoption of EDC and ePRO is not just a technological upgrade but a critical step in refining clinical research methodologies. As reported in Contemporary Clinical Trials, the shift towards electronic methods addresses barriers such as low technology adoption in resource-limited settings and the need for standardized selection and administration of Patient Reported Outcome (PRO) measures. The path to efficient information control is intricate, requiring meticulous strategizing to prevent economic consequences resulting from less than optimal choices. The process requires careful attention, from information extraction to conversion and migration, to meet the distinct requirements of health systems.
Essentially, the progress of information control through EDC and ePRO solutions is a sign of the wider move towards a more precise, effective, and patient-focused method to scientific investigation, demonstrating a dedication to utilizing technology for the improvement of health results.
Challenges and Solutions in Managing Large Volumes of Data
In the domain of research in the medical field, the rapid expansion of information necessitates robust systems for handling and organizing research findings. Contract Research Organizations (CROs) specializing in clinical data management are leveraging cloud-based solutions, advanced analytics, and machine learning to streamline the handling of extensive datasets. These technologies are crucial in analyzing and understanding the intricate information landscape, enabling CROs to obtain valuable conclusions that drive healthcare progress.
One illustrative case involved a NIH-funded medical school in Florida, which transitioned to an independent ServiceNow environment for enhanced control over its healthcare operations. The capacity to distinguish and merge between their scholarly and healthcare fields was vital to their operational achievement, emphasizing the significance of customized information administration solutions.
Similarly, Ambience, a nimble healthcare company, partnered with Redox to leverage their proficiency in healthcare interoperability standards. This strategic decision enabled Ambience to concentrate on refining their product offerings instead of being bogged down by individual integration challenges with every new client.
In another example, LLR PCL, a community interest company, sought to improve their IP&C auditing process. They substituted labor-intensive paper forms and spreadsheets with a centralized system, lessening risks of information mismanagement and loss, and improving the reliability of incident reporting.
These cases highlight the importance of having advanced information management tools that are skilled at handling the specific requirements of healthcare entities. As the volume of healthcare information continues to expand at an astonishing rate—accounting for approximately 30% of the world's volume of information with an annual growth of 36% by 2025—such tools become indispensable. This growth not only advances research in the field of medicine and pharmaceutical development but also enhances the operational efficacy of healthcare providers.
Moreover, considering the projected substantial revenue that the United States is expected to generate in the market of technology for healthcare, the possibilities for advancement in management and analysis of information are vast. The incorporation of information into healthcare is not without its challenges, as demonstrated by the difficulties in accessing and utilizing unstructured data, such as medical images and notes. Nevertheless, the capability to methodically control and examine this information is essential for leveraging the complete potential of AI in healthcare.
In summary, the challenges presented by the vast volumes of information in healthcare are met with innovative information management strategies that consider the unique requirements of various healthcare sectors. These strategies not only support the operational needs of healthcare providers but also contribute significantly to enhancing patient care and outcomes.
The Importance of Data Quality and Compliance in Clinical Trials
The reliability of trial results depends heavily on the quality and adherence of the information gathered. Contract Research Organizations (CROs) that specialize in clinical information oversight have been given the crucial responsibility of ensuring the excellence and compliance with regulatory standards of this data. These organizations utilize extensive information management systems, perform thorough quality assessments, and adhere to stringent regulatory guidelines to safeguard the integrity of the data. The consequence of substandard information is substantial; poor quality data can lead to the overlooking of vital patterns or relationships, particularly in the context of Machine Learning (ML) and Artificial Intelligence (AI) where the precision of information is paramount.
On the frontline of innovation, companies like QuantHealth are revolutionizing the way clinical trials are conducted by integrating AI and cloud technology, thus streamlining the process, enhancing efficiency, and elevating the probability of success in drug development. Similarly, Truveta's dedication to high-quality information gathering is apparent in their significant investment in workflow support for regulatory submissions and audit-ready processes. This commitment to the quality of information is not just about meeting current needs but also about preparing for a future where real-world evidence plays a pivotal role in regulatory submissions.
Moreover, the arrangement of trials, which are classified into phases, each with distinct objectives, highlights the need for rigorous information control. From the initial phase involving a few volunteers to subsequent phases that evaluate effectiveness and safety in larger populations, the information gathered at each stage is vital for drawing dependable conclusions about new interventions in healthcare.
In the ever-changing field of medical studies, the utilization of real-life information has gained newfound significance, as witnessed during the COVID-19 outbreak when swift advancements were crucial. The FDA's acceptance of real-world information from sources like medical device registries for certain reporting requirements indicates a change towards embracing diverse sources for regulatory decisions.
To utilize the complete potential of the extensive information produced from trials, it is crucial to implement a strategic approach from the beginning. This involves identifying the ideal sources of information—ranging from conventional to digital—and the methods for handling the flow of this information. The ultimate goal is to derive profound insights and trends that can inform smarter, safer, and more patient-centric drug development decisions.
Future Trends in Clinical Data Management: Machine Learning and Data Warehousing
The integration of machine learning and big analytics is revolutionizing the field of clinical management. With a staggering 91.5% of companies investing in machine learning and AI, the medical research landscape is experiencing a transformation, leading to improved patient outcomes and more personalized care. Machine learning algorithms, in particular, are instrumental in deciphering intricate datasets, offering insights that might elude human analysis. These algorithms can predict patient outcomes with remarkable accuracy, enhancing diagnostic processes and treatment plans.
Moreover, warehousing solutions for information are advancing to accommodate the extensive quantities of information generated in healthcare. This organized approach to information storage not only enables accessibility but also guarantees that information remains structured for efficient retrieval and analysis. As the healthcare industry deals with a significant influx of unstructured information, from patient records to medical histories, advancements in AI offer a beacon of hope. Generative AI, for instance, permits the efficient comparison of documents, streamlining operations and reinforcing service productivity.
Innovative services like Amazon Bedrock and AWS are at the forefront, providing the architecture necessary for implementing these advanced solutions. They demonstrate how technology can be utilized to address the healthcare industry's challenges in managing information, transforming extensive and intricate datasets into practical insights. With the ongoing progress of technology, CROs specializing in medical research are in a strong position to capitalize on these trends, opening up new avenues for advancement in patient care.
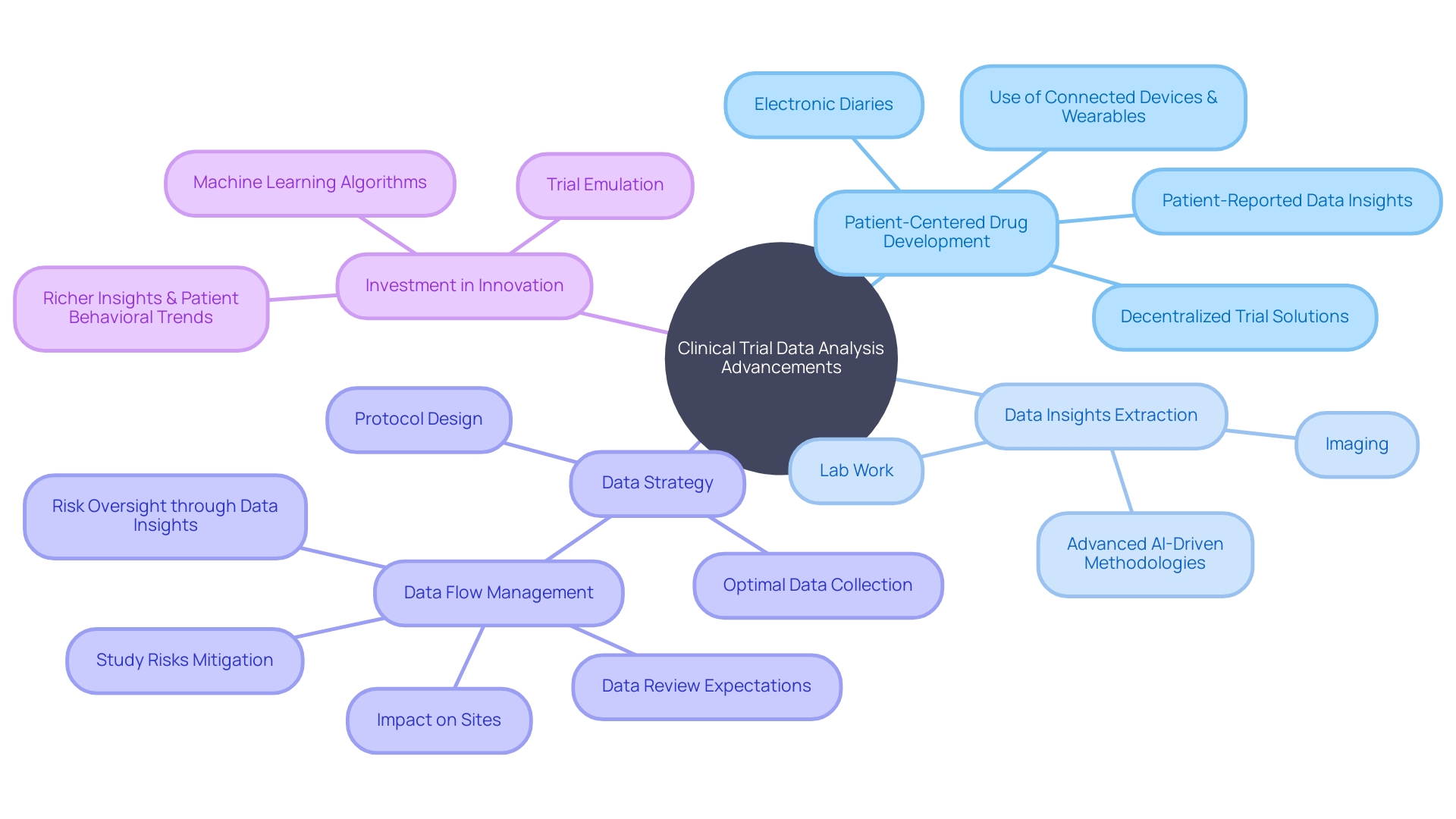
Case Study: How CROs Are Leveraging Data Science to Enhance Clinical Trials
A CRO that focuses on organizing and analyzing information for trials has recently demonstrated the transformative impact of science on trials. By using advanced analytical tools and machine learning algorithms, they were able to uncover crucial trends and patterns in trial information, which directly facilitated more detailed and patient-specific treatment approaches. This advancement is proof of the vital role that management CROss play in the integration of science for the improvement of medical research.
In the realm of clinical trials, the use of connected devices, wearables, electronic diaries, and other decentralized trial solutions is increasingly prevalent, thereby amplifying the volume and variety of information. The wise use of artificial intelligence allows the extraction of valuable insights from multifaceted sources such as lab results, patient-reported outcomes, and imaging. This comprehensive analysis of information not only illuminates patient behaviors but also supports more enlightened decision-making in drug development. A deliberate approach to handling this overwhelming amount of information is crucial, requiring a premeditated strategy ahead of protocol development.
Furthermore, the notion of 'trial emulation' has surfaced as a powerful tool in evaluating treatment effectiveness through the analysis of available information, thereby diminishing the need for conducting multiple clinical trials. Innovations like the HINT algorithm from the University of Illinois Urbana-Champaign provide predictive insights into the potential success of a trial, influencing modifications in trial design or drug selection.
These advancements highlight the story that while information is plentiful, the true challenge lies not in its accumulation but in its meaningful interpretation. As highlighted by industry experts, investment in innovation must be prioritized to achieve a deep, causal, and contextual understanding of human information, ultimately connecting genetic markers to specific disease states. This evolution in data management and analysis heralds a new era of patient-centered drug development, where safety, efficacy, and personalized healthcare are at the forefront.
Conclusion
In conclusion, the field of clinical data management is evolving with the integration of advanced technologies. AI-powered algorithms, Clinical Data Repositories (CDRs), Electronic Data Capture (EDC) systems, and electronic Patient Reported Outcomes (ePRO) are revolutionizing healthcare by providing real-time, patient-centered information and improving treatment decisions.
Managing large volumes of data is a challenge, but innovative strategies are being developed. Cloud-based solutions, advanced analytics, and machine learning streamline data handling, leading to insightful conclusions that drive medical advancements. Ensuring data quality and compliance in clinical trials is crucial, with Contract Research Organizations (CROs) playing a vital role in safeguarding data integrity.
Looking ahead, the integration of machine learning, data warehousing, and data science will continue to transform clinical data management. Machine learning algorithms enhance diagnostic processes and treatment plans, while data warehousing facilitates efficient data organization and accessibility. These advancements unlock new possibilities for medical research and improve patient care.
In summary, the evolution of clinical data management is driven by advanced technologies, leading to improved patient outcomes and personalized care. Strategic planning, data quality, and compliance are essential for harnessing the full potential of data in medical research. The future holds immense potential for innovation in data management and analytics, further propelling the field of clinical data management towards a patient-centric approach and continuous improvement in healthcare outcomes.




