Introduction
Clinical trial companies play a crucial role in advancing medical knowledge and improving patient care. These companies meticulously conduct research programs to determine the effectiveness of new interventions and treatments.
Clinical research consultants, experts in the field, are integral to the success of these trials. They provide specialized expertise in study design, statistical analysis, and navigating complex regulatory landscapes.
This article explores the role of clinical research consultants in clinical trials, from study design and protocol development to participant recruitment and data collection. It also highlights the importance of ethical considerations and regulatory compliance in ensuring the integrity of clinical trials. By understanding the vital contributions of clinical research consultants, we can appreciate the significance of their work in driving medical advancements and improving patient outcomes.
Background and Context
Clinical trial companies are the linchpins in the realm of medical advancements, meticulously orchestrating research programs to ascertain the superiority of novel interventions over existing treatments. These trials are pivotal around the globe, including groundbreaking research on lung cancer treatments pending regulatory endorsement.
The investigational therapies undergoing these trials often stem from prior research demonstrating potential efficacy. The diversity of clinical trials extends to evaluating new uses for approved drugs, assessing treatments in treatment-naive patients, and exploring options for individuals who have not responded to existing therapies.
Kaplan, a clinical research connoisseur, emphasizes that FDA approval is a testament to a drug's safety and efficacy, historically contingent upon at least two robust clinical studies. Nonetheless, the 21st Century Cures Act of 2017 has recalibrated these criteria, allowing for a single trial to suffice under certain conditions.
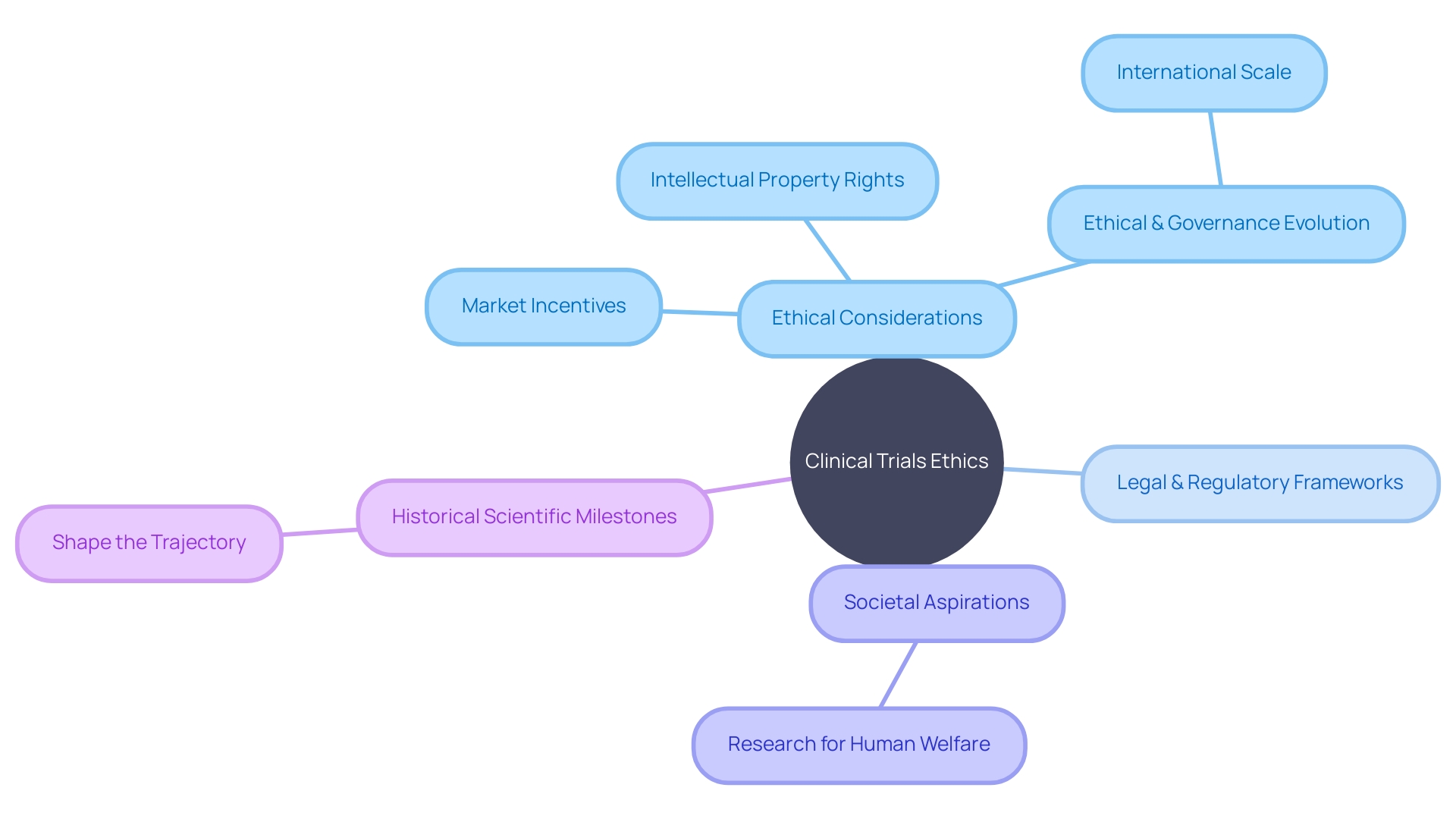
This shift reflects a broader spectrum of ethical, legal, and social considerations inherent in emerging medical technologies. These considerations encompass market incentives, intellectual property rights, and the technology's ethical and governance evolution on an international scale. The legal and regulatory frameworks, societal aspirations of research aimed at enhancing human welfare, and the historical scientific milestones all interweave to shape the trajectory of clinical trials. With a focus on the ethical dimensions, clinical trial companies navigate through a complex tapestry of factors to ensure the research aligns with both scientific rigor and societal values.
The Role of Clinical Research Consultants
Clinical research consultants are integral to the clinical trial process, providing specialized expertise that drives the success of medical research initiatives. With the advent of organizations like CMIC Group, which pioneered the Contract Research Organization (CRO) in Japan, the landscape of clinical trials has evolved significantly.
These consultants are tasked with crucial responsibilities that include designing robust studies, crafting detailed statistical analysis plans, and ensuring that the trials navigate the complexities of regulatory landscapes across different countries. For instance, their expertise becomes particularly vital in scenarios where patients from remote locations, such as rural Pennsylvania, are involved in international trials, such as those in Turkey.
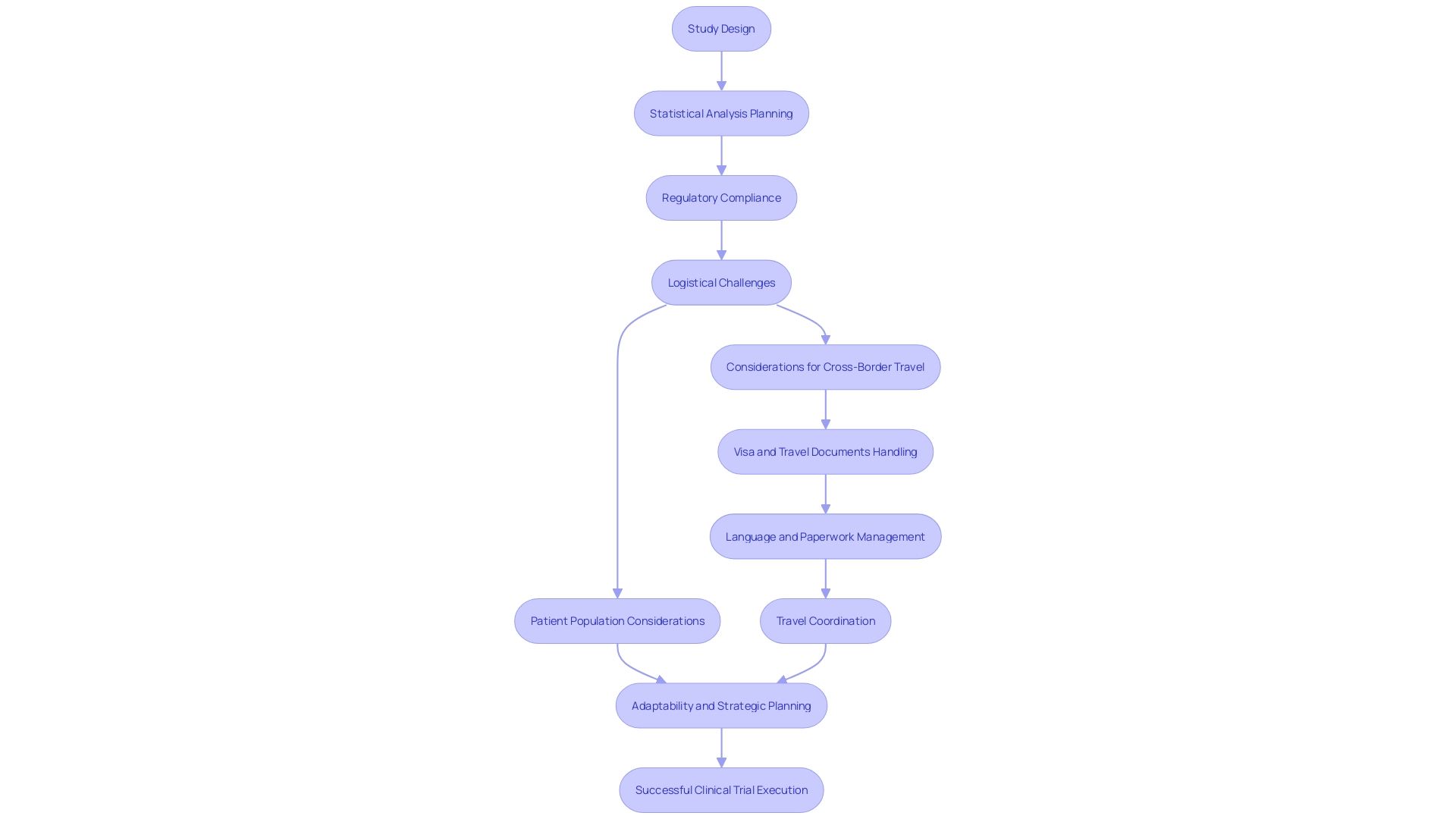
They must address the multitude of logistical challenges, from obtaining visas to handling paperwork in foreign languages. This underscores the importance of meticulous study design and preemptive planning to mitigate potential issues like bias and confounding factors. The role of the epidemiologist is highlighted by a focus on critical thinking in study design to answer pivotal questions while preventing or addressing study pitfalls. It is this level of strategic planning and foresight that clinical research consultants bring to the table, ensuring that clinical trials are not only compliant but also adaptable to the needs of diverse patient populations.
Study Design and Protocol Development
Clinical research consultants collaborate with clinical trial companies to design studies and develop protocols that adhere to ethical and regulatory guidelines. They help determine the appropriate study population, intervention, and outcome measures, ensuring that the research objectives are clearly defined.
Regulatory Compliance
Upholding the integrity of clinical trials is paramount, as these studies are critical in establishing the safety and efficacy of new medical treatments. A pivotal player in this process is the Contract Research Organization (CRO), exemplified by pioneers like CMIC Group, which has been at the forefront of the CRO industry in Japan for over three decades. These organizations specialize in offering end-to-end solutions that span the entire pharmaceutical value chain, ensuring that each phase of drug development, from initial conception to market entry, meets the stringent standards set forth by regulatory bodies.
The rigorous oversight by CROss encompasses compliance with Good Clinical Practice (GCP) and International Conference on Harmonization (ICH) guidelines, which are essential for the protection of participant rights and safety. By obtaining necessary approvals from regulatory authorities and ethics committees, CROss like CMIC facilitate the progression of clinical trials through their various stages—from the initial trials with healthy volunteers, aimed at assessing safety, to later phases that recruit patients to evaluate treatment efficacy and safety. This structured approach ensures that new interventions, whether drugs, devices, or behavioral tactics, are not only safe for patient use but also offer improved effectiveness or reduced side effects compared to existing treatments.
Participant Recruitment and Informed Consent
The landscape of clinical trials is evolving with an increased focus on patient-centricity, ensuring the experience of participants is at the forefront of study planning and design. Daniel J. Herron, a leading figure in regulated industries, emphasizes the importance of involving patients early on to ensure their perspectives and needs shape the trial.
This approach includes clear communication, with information presented in an easily digestible format for participants. A diverse participant pool is essential to reflect the varying responses to diseases across different demographics, enhancing the relevance and equity of the research.
Ethical considerations are also paramount, with recent shifts advocating for fair treatment of participants through adequate compensation. This aligns with the principle that contributions to public health advancements, like those of first responders, should be recognized and valued.
Compensation not only acknowledges the participant's time and potential risks but also helps cover associated expenses, aligning with the ethics of respect for individual rights and dignity. Statistics illustrate the complexities faced by participants, such as a rural Pennsylvania patient with an ultra-rare disease contemplating a clinical trial in Turkey. The logistical challenges of international travel for clinical trials bring to light the need for comprehensive support systems for participants, ensuring that they can fully engage in the trial without undue burden. This scenario underscores the necessity of addressing both the practical and ethical aspects of patient participation in clinical research.
Data Collection and Management
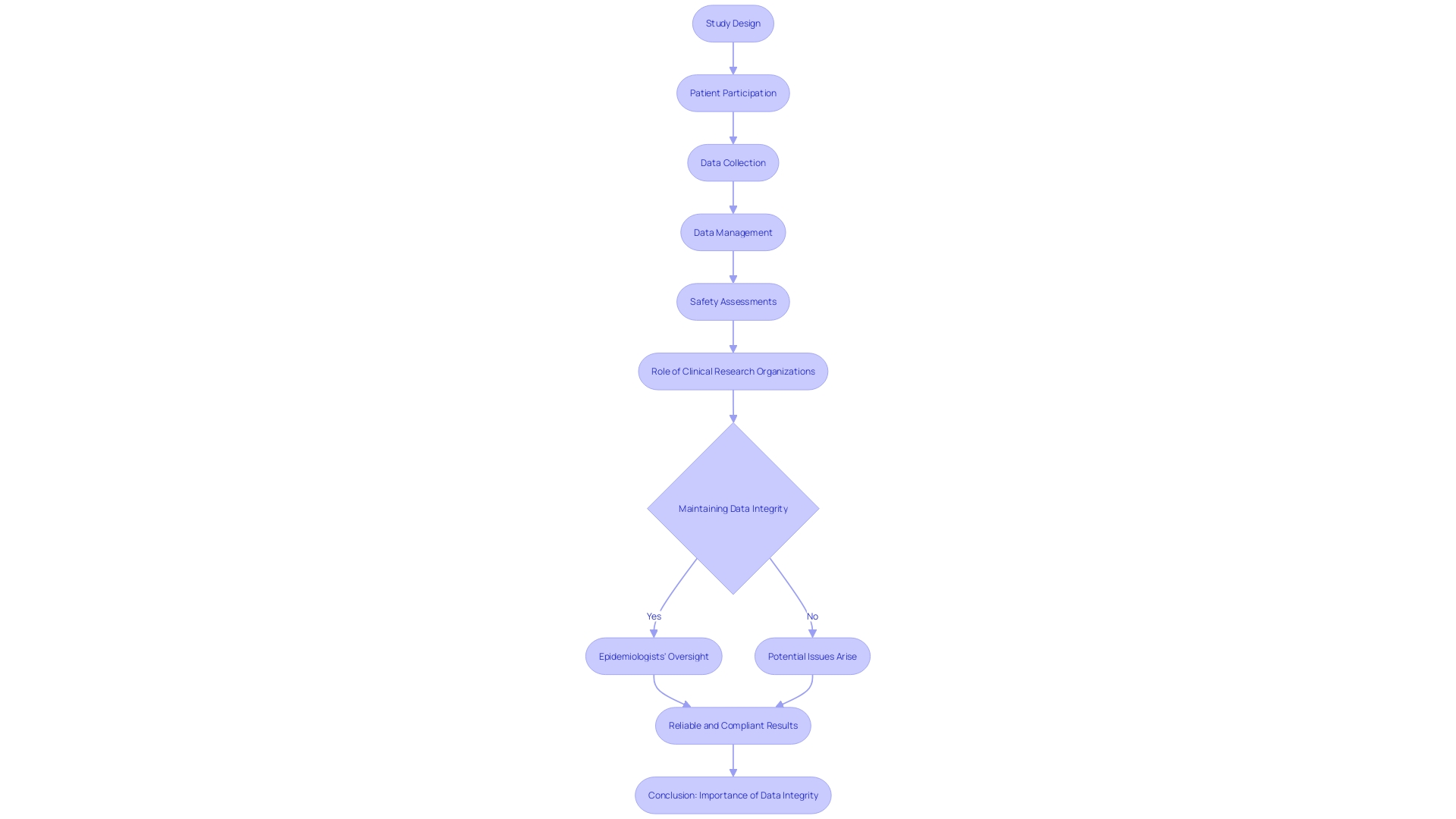
In the realm of clinical trials, the integrity of data is paramount. Not only must researchers contend with the complexities of study design and analysis, but they also face the practical challenges of patient participation.
For instance, a patient from rural Pennsylvania must navigate international travel to join a trial in Turkey, dealing with visas, language barriers, and unfamiliar documentation, all for a potentially lifesaving treatment. Epidemiologists are essential in ensuring that study designs are robust enough to yield reliable answers to medical questions.
Their critical thinking is crucial to designing studies that preempt issues like bias and confounding factors, or to address them if they arise. Clinical research consultants play a vital role in maintaining data integrity.
They develop precise data collection forms, conduct comprehensive training on data collection procedures, and implement sophisticated data management systems in line with quality standards and data protection laws. Their meticulous work in safety assessments involves not just recording adverse events but also complex statistical analyses that incorporate data from multiple trials, treatment discontinuations, and the definition of incidence measures to accurately assess risks and benefits. In light of these challenges, bioaccess™ offers a tailored solution with cost-effective, high-quality clinical research organization (CRO) services in Latin America. The clinical research team at bioaccess™, with over 20 years of experience in medtech, specializes in conducting a range of studies including pilot, first-in-human (FIH), early-feasibility (EFS), pivotal, and post-market clinical follow-up (PMCF) studies. Their expertise ensures that medical devices are advanced efficiently and effectively, addressing the needs of clinical research to deliver reliable and compliant results.
Monitoring and Quality Assurance
Ensuring the integrity of clinical trials is paramount, a responsibility that falls significantly on the shoulders of clinical research consultants. These professionals are tasked with conducting thorough monitoring visits to various trial sites.
Their role is critical in verifying that each site strictly adheres to the study protocols, complies with both regulations and ethical standards, and maintains the accuracy of collected data. This rigorous oversight is essential, particularly in light of the fact that clinical trials serve as the foundation for establishing the safety and efficacy of new treatments or interventions.
As the first and largest Contract Research Organization in Japan, CMIC Group has led the industry in providing end-to-end solutions, emphasizing the necessity of high standards in trial conduct. These trials, which may range from small Phase one studies focusing on treatment safety to larger Phase two trials assessing efficacy, are crucial in shaping patient care and outcomes. It's the consultant's duty to assess participant safety vigilantly, pinpoint any deviations from the protocol, and implement robust quality assurance measures. This diligence ensures the reliability of study data, which is instrumental in determining whether new treatments can be deemed safe and effective for patient use.
Advancing Medical Knowledge
Clinical research consultants play an indispensable role in the realm of medical advancements. By offering specialized expertise and unwavering support, they are fundamental in ensuring that clinical trials adhere to the highest standards of scientific rigor and ethical practice.
Their contributions are critical, from navigating the complexities of protocol compliance to enhancing the accuracy and dependability of clinical data. For instance, in the face of logistical challenges, such as a patient needing to travel internationally for a trial, consultants provide essential guidance to facilitate participation while mitigating the stress of managing cross-border travel and documentation.
Moreover, organizations like CMIC Group, which blazed the trail for Contract Research Organizations in Japan, exemplify the industry's evolution. They deliver an extensive suite of services that cover the full spectrum of drug development, thereby enabling a seamless transition from concept to commercialization. Such organizations not only offer end-to-end solutions but also adapt to meet the specific needs of their clients—be they pharmaceutical firms, medical device manufacturers, or academic institutions—thereby propelling forward the development of novel and efficacious treatments.
Case Study Examples
Clinical research consultants play a pivotal role in bridging the gap between medical advancements and patient accessibility. In rural Pennsylvania, for instance, a patient with an ultra-rare disease is presented with the chance to join a clinical trial in Turkey.
The complexity of international travel, language barriers, and document management poses significant challenges. This scenario underscores the value of clinical research organizations (CROs), such as CMIC Group, which has been an industry leader for over 30 years.
CMIC has innovated the concept of end-to-end solutions, providing comprehensive services that cover the entire pharmaceutical value chain. Their expertise in contract development, manufacturing, and navigating market entry is crucial for patients and medical institutions alike, ensuring that the path from clinical research to treatment is navigable and efficient. An example of such expertise can be seen in the collaborative efforts within funding programs that aim to digitize transportation systems, highlighting the importance of teamwork in overcoming complex, multifaceted challenges. Furthermore, the story of a clinician like Danielyan, who transitioned from pediatric practice to clinical research, illustrates the personal and professional growth that can be achieved through continued education and the support of robust clinical research frameworks.
Case Study 1: Investigating a New Cancer Treatment
A clinical trial company, in partnership with a seasoned clinical research consultant, embarked on an investigation to assess the effectiveness and safety of a groundbreaking treatment for cancer. The consultant was instrumental in crafting the study's protocol, ensuring adherence to regulatory standards, and overseeing the integrity of the data collected.
The outcome of the study yielded promising results, indicative of the potential for refining cancer therapies. This case illustrates the complexities and critical importance of meticulous planning and execution in clinical trials, particularly when considering the challenges faced by patients with rare diseases who may need to travel internationally for participation.
The logistical hurdles, such as obtaining visas, navigating foreign languages for paperwork, and coordinating travel, underscore the necessity for thorough preparation and patient support. As experts reflect on the evolution of clinical trial management, it is evident that strategic foresight can significantly influence outcomes. The practice of 'bulletproofing' decisions, optimizing each step of the process, and starting preparations early are pivotal in addressing the unique requirements of rare disease research, where funding and comprehensive approaches are still developing. This case study not only highlights the potential advancements in cancer treatment but also the broader implications for managing complex clinical trials with the utmost care and consideration for all participants.
Case Study 2: Assessing the Effectiveness of a Medical Device
A clinical trial company recently embarked on a pivotal project to evaluate the effectiveness of an innovative medical device aimed at treating a particular health condition. With the support of a seasoned clinical research consultant, the trial was meticulously planned and executed.
The consultant's role was instrumental in the study design, participant recruitment, and the management of data collection and analysis. The results of the study were promising, showcasing the medical device's potential and paving the way for its integration into clinical settings. These findings hold significant implications for the company, potentially leading to successful business outcomes such as acquisition by a larger entity, going public through an IPO, or entering lucrative licensing partnerships—all of which are markers of success in the medical device startup arena.
Methodology and Approach
In the pursuit of advancing medical knowledge, the significance of well-designed clinical studies cannot be overstated. Our case study delved into the intricacies of clinical supervision by harnessing a qualitative research approach, gathering rich data through extensive interviews with clinical research consultants, representatives from clinical trial companies, and study participants. The thematic analysis illuminated the nuanced roles these professionals play in the epistemic work of clinical trials, revealing how they enact their expertise in the tangible actions of designing and overseeing studies.
This echoes the sentiments of epidemiologists who underscore the critical nature of meticulous study design and statistical planning to address the right questions and mitigate potential mishaps. With over 30 years of innovation, CMIC Group, Japan's foremost Contract Research Organization, exemplifies the industry's drive to provide comprehensive solutions that span the entire pharmaceutical value chain. From development and manufacturing to market entry strategies, organizations like COMIC are pivotal in streamlining the journey of drug development, ensuring that each phase is executed with precision and tailored to the unique needs of their clients, thereby safeguarding the integrity of clinical research and its contribution to patient outcomes.
Results and Outcomes
Clinical trial companies, such as Contract Research Organizations (CROs), have become integral to the advancement of medical treatments by providing comprehensive services that span the full spectrum of the drug development process. With over three decades of innovation, companies like CMIC Group have established themselves as industry leaders, offering a suite of services that includes study design, regulatory compliance, participant recruitment, data collection, and monitoring. These organizations are pivotal in bridging the gap between clinical trials and clinical practice, addressing challenges such as the translation of trial results into real-world applications.
As noted in a recent JAMA publication, despite the registration of approximately 40,000 randomized controlled trials (RCTs) annually on ClinicalTrials.gov, there remains a significant disconnect between the narrowly defined parameters of RCTs and the broad context of patient care. CROs are positioned to mitigate these disparities by ensuring that the design and conduct of trials align more closely with clinical needs. Moreover, CROss like CMIC provide end-to-end solutions, meeting the specific needs of pharmaceutical companies, medical device manufacturers, and research institutions, thus facilitating the progression of products from conception to market entry and beyond.
Discussion and Implications
Clinical trial companies, as evidenced by recent case studies and industry movements, are at the forefront of bridging the divide between research and patient-centric care. A poignant example is the ordeal faced by a Pennsylvanian patient suffering from an ultra-rare disease, who must navigate the complexities of international travel to participate in a clinical trial in Turkey.
The logistical challenges such as obtaining visas, managing non-native language documents, and coordinating travel underscore the patient and family's need for support that extends beyond the clinical aspect of trials. This real-world scenario parallels the discussions at the inaugural JAMA Summit, where experts recognized the silos between clinical trials and practice.
The JAMA paper highlighted the inefficiencies and limitations that arise when trials are narrowly defined, failing to encompass the broader clinical context. With over 40,000 RCTs registered annually on ClinicalTrials.
Gov and many treatment guidelines grounded in expert opinion rather than high-quality RCTs, the integration of clinical trials into real-world practice is imperative. Contract Research Organizations (CROs) like CMIC Group, which pioneered the CRO business in Japan over 30 years ago, have evolved to offer end-to-end solutions that address these very challenges. By providing services that span the pharmaceutical value chain, including development, manufacturing, and market entry solutions, CROss meet the needs of various stakeholders, including patients, pharmaceutical companies, and medical institutions. As the largest CRO in Japan, CMIC exemplifies the industry's commitment to innovation and patient-centric solutions, ensuring that clinical trials not only advance medical knowledge but also directly impact patient care and outcomes.
References
Clinical research consultants play a pivotal role in the advancement of medical knowledge. Their expertise in study design and statistical analysis is critical in addressing the right questions and ensuring the accuracy and integrity of clinical trials.
With a deep understanding of how to mitigate biases, confounding factors, and other potential issues, these consultants are essential in crafting robust research studies that yield reliable data. For over three decades, Contract Research Organizations (CROs) like CMIC Group have been at the forefront of the industry, providing comprehensive services across the entire pharmaceutical value chain.
CMIC, Japan's inaugural and largest CRO, has been a pioneer in delivering customized solutions for a diverse clientele, including pharmaceutical companies, medical device manufacturers, academic institutions, bio-ventures, and medical facilities. Emphasizing the significance of a well-structured study, one epidemiologist highlighted, 'Much of my training has to do with thinking critically about how to design a study to answer the right question... How do we account for bias, confounding factors, and other important issues that we worry about in designing human studies?' This meticulous approach to clinical research underscores the essential nature of these consultants' roles in the successful execution of clinical trials.
Conclusion
In conclusion, clinical research consultants are integral to the success of clinical trials. They provide specialized expertise in study design, statistical analysis, and regulatory compliance.
By ensuring adherence to ethical guidelines and regulatory standards, they maintain the integrity of trials and protect participant rights. These consultants play a crucial role in developing robust protocols, determining appropriate interventions and outcome measures.
They also prioritize patient-centricity by promoting clear communication, diverse participant recruitment, and fair treatment. Their meticulous approach to data collection and management ensures data integrity and accurate assessment of risks and benefits.
The contributions of clinical research consultants drive medical advancements and improve patient outcomes. Contract Research Organizations like CMIC Group exemplify the industry's commitment to providing comprehensive solutions throughout the drug development process.
They adapt to meet the specific needs of clients across the pharmaceutical value chain. By understanding the importance of clinical research consultants, we can appreciate their significant impact on advancing medical knowledge while prioritizing patient safety and welfare. Their expertise drives scientific rigor, ethical practice, and adaptability to diverse patient populations. In summary, clinical research consultants are vital in driving the success of clinical trials by providing specialized expertise and ensuring adherence to ethical guidelines. Their contributions shape the trajectory of medical advancements, improving patient care globally.




