Introduction
Clinical trials consultants play a vital role in bridging scientific discovery with clinical application by providing oversight and expertise throughout the research process. Their involvement ensures that trials adhere to regulatory standards, employ advanced technologies effectively, and optimize operational efficiencies. This article explores the responsibilities of clinical trial consultants, their qualifications and skills, and the benefits of utilizing their services.
By understanding the significance of these professionals in shaping the trajectory of medical research, we can appreciate their invaluable contributions to patient care outcomes and healthcare innovation.
The Role of Clinical Trials Consultants
Clinical trials consultants are pivotal in merging scientific discovery with clinical application, offering a blend of expertise that catalyzes progress in medical research. These specialists provide critical oversight to trials, ensuring that from the initial design to the final execution, each stage adheres to regulatory standards and best practices. With a keen eye for balanced trial design and a commitment to deploying advanced technologies effectively, consultants help safeguard the integrity of the research process.
They navigate complex regulatory landscapes, ensuring compliance while optimizing operational efficiencies. The involvement of these professionals is not just procedural—it has tangible impacts on patient care outcomes and healthcare innovation.
Contemporary clinical trial design acknowledges the necessity of impeccable scientific inquiry coupled with a streamlined operational approach. As observed by industry expert Ken Getz, the shift towards integrating sophisticated technologies like electronic data capture signifies advancement within the field. Yet, consultants remain aware of the potential burdens these technologies can pose, ensuring their implementation enhances rather than hinders the research process.
The significance of this meticulous approach is evident in cases involving complex logistics, such as coordinating international participation in trials, where a consultant's guidance is indispensable for managing cross-border challenges.
Clinical trials, while inherently focused on scientific rigor, also profoundly impact patient experiences and outcomes. Trials are meticulously staged—initiating with a small cohort of healthy volunteers to assess safety, advancing to larger groups to evaluate efficacy, and only then confirming general safety and effectiveness for patient use. Through this evolution, consultants play an essential role in sculpting the blueprint of trials, ensuring each phase generates valid and useful data.
This operational wisdom, as highlighted in the insights shared by Treehill's advisory services, can determine the trajectory of a treatment's development years in advance. By forecasting and optimizing decision-making early on, consultants can exponentially improve the eventual outcomes of clinical research.
Moreover, the narratives of clinical research professionals, like that of the clinical research technician, underscore the passion and dedication underlying these roles. Whether aiding in the development of life-saving treatments, such as the polio vaccine, or guiding cutting-edge research, consultants facilitate the invaluable contributions of volunteer participants. Their involvement ensures the success of each study, driving forward the mission of enhancing health care for all.
Key Responsibilities of Clinical Trials Consultants
Clinical trial consultants leverage their expertise to craft solid research protocols, ensuring that they are clear, ethical, and robust. They expertly navigate regulatory frameworks to secure the required approvals, aligning the study with essential guidelines. Through a meticulous assessment of study feasibility, they factor in patient availability, site capabilities, and necessary resources, preempting challenges to set realistic timelines and budgets.
At the heart of site selection, they scrutinize and manage research locations, providing continual support to site staff. Risk mitigation is paramount, as they anticipate and counteract potential issues, safeguarding trial integrity and participant well-being. Their analytical prowess extends to data scrutiny, where they help distill significant findings, and create detailed reports that propel the dissemination of new medical insights.
These professionals also act as the vital link between varied stakeholders, ensuring cohesive efforts and the diffusion of the latest industry developments. Their commitment extends to the evolving landscape of clinical research, exemplified by the role China's clinical research nurses play in coordinating projects and implementing rigorous training programs. Such dedicated teams ensure high-quality research outcomes, as seen in the structured, expert-driven approach adopted by the International Association of Clinical Research Nurses' Shanghai Chapter.
In contemporary practice, they also confront new logistics, like guiding a patient from rural Pennsylvania to an international clinical trial or harnessing technology to facilitate trial participation remotely. With a keen eye on market trends, they acknowledge the growing significance of Contract Research Organizations (CROs) like IQVIA, a standout in a booming $77 billion industry projected to expand significantly. There is an evident shift towards innovation in MedTech, where companies like Archetype work to smooth the journey of medical devices from conception to market approval, addressing the complex regulatory landscape.
The role of trial consultants reflects a dynamic fusion of strategic foresight, regulatory acuity, and an unwavering commitment to improving outcomes, underpinned by various entities from research teams composed of dedicated individuals like Chris, a biomedical engineer with experience in pivotal studies, to the concerted efforts of organizations urging public involvement in clinical research for the betterment of healthcare worldwide.
Qualifications and Skills Required
Navigating the multifaceted world of clinical trials requires a unique blend of scientific acumen, regulatory savviness, and project leadership. These consultants, often with advanced degrees in medicine, pharmacy, or life sciences, are essential for steering clinical studies in line with stringent regulatory frameworks to safeguard participant well-being and satisfy quality standards.
Consultants must possess comprehensive understanding of guidelines at each clinical trial stage to maintain integrity and compliance. For instance, Phase one trials prioritize safety with just a handful of volunteers, while Phase two expands participation and assesses treatment efficacy.
Effective management of clinical trial components, such as meticulous scheduling, resource allocation, and diligent monitoring, falls within their realm, aligning with the model of Contract Research Organizations (CROs) like CMIC Group. With services spanning the drug development spectrum—including manufacturing and market entry solutions—CROss demonstrate that adaptable, client-centered approaches are keys to pushing medical innovations forward.
Moreover, exceptional communication skills are non-negotiable for liaising with diverse teams and stakeholders. This is evident in scenarios where a patient needs to travel for an investigational treatment; a clinical trials consultant would play a pivotal role in simplifying cross-border complexities. They facilitate conversations around logistical hurdles, fostering an environment that integrates the 'patient voice' in decision-making, as highlighted by recent initiatives like the MHRA's Patient Involvement Strategy.
Strong analytical and problem-solving skills allow these professionals to discern and resolve unforeseen challenges, bolstering the trial's progression. Their attention to detail underpins accuracy in all research stages, and they proudly uphold ethical standards to ensure the rights and safety of research participants are never compromised.
Spanning from-bedside-to-bench, the consultants' contributions are central to enhancing patient outcomes and optimizing healthcare systems, ultimately contributing to a thriving research culture within the medical community.
Benefits of Utilizing Clinical Trials Consultants
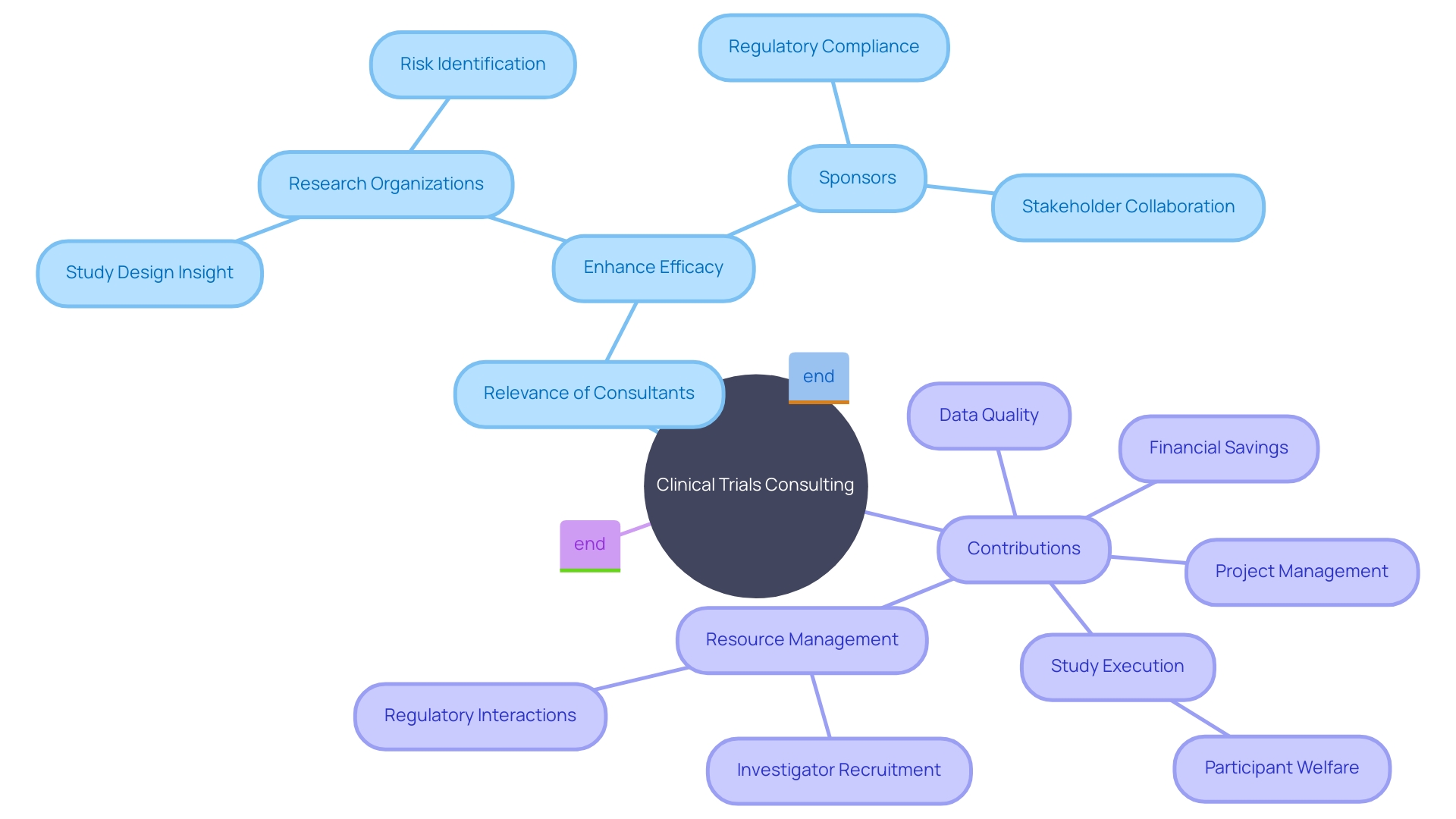
Engaging clinical trials consultants can significantly enhance the efficacy of research organizations and sponsors in numerous ways. These specialists proffer pivotal insights and guidance to foster optimized study design and effective execution. Compliance with stringent regulations ensures high integrity and quality of research, fostering reliable outcomes.
Efficient implementation hinges on recognizing potential hurdles, formulating pragmatic timelines, and smart resource distribution, a process streamlined by consultants. Risk identification and sound mitigation strategies are crucial, safeguarding participant welfare and the robustness of data collected during studies.
Consultants serve as conduits to expansive networks and resources, expediting investigator recruitment and site selections while smoothing interactions with regulatory entities. Their intervention strengthens stakeholder collaboration, vital for clear communication and coordination. Project management acumen offered by consultants is instrumental in refining procedures, judiciously deploying resources, and achieving key milestones, promoting productivity and efficiency.
Moreover, strategic utilization of these consultants can result in financial savings by foreseeing and circumventing expensive oversights, curtailing setbacks, and bettering study results. Ultimately, these consultancies are indispensable allies in fostering the progression of medical science within clinical research domains.
Conclusion
In conclusion, clinical trials consultants are invaluable in bridging scientific discovery with clinical application. They ensure trials meet regulatory standards, utilize advanced technologies effectively, and optimize operational efficiencies. By overseeing trial design and navigating complex regulations, consultants safeguard the research process's integrity, resulting in improved patient care outcomes and healthcare innovation.
The responsibilities of clinical trials consultants include crafting research protocols, securing approvals, assessing feasibility, managing locations, mitigating risks, analyzing data, and facilitating collaboration among stakeholders. These professionals adapt to evolving logistics and market trends, such as remote trial participation and MedTech innovation, enhancing the efficiency of clinical research.
To excel in their roles, clinical trials consultants need scientific acumen, regulatory knowledge, and project leadership skills. Effective communication, analytical thinking, problem-solving, and attention to detail are crucial for collaborating with diverse teams and resolving challenges. Their contributions improve patient outcomes, uphold ethical standards, and optimize healthcare systems.
Engaging clinical trials consultants provides numerous benefits to research organizations and sponsors. Their guidance enhances study design and execution, ensuring compliance and high-quality research outcomes. By identifying and mitigating risks, consultants protect participant welfare and data reliability.
Additionally, their project management expertise and extensive networks expedite recruitment, site selection, and stakeholder collaboration. Strategic utilization of clinical trials consultants leads to financial savings, improved results, and the advancement of medical science in clinical research.
In summary, clinical trials consultants play an essential role in shaping the trajectory of medical research. Their expertise and oversight drive the success of trials, impacting patient care outcomes and fostering healthcare innovation. Through their qualifications and skills, consultants ensure compliance, collaboration, and optimization.
By utilizing the services of these professionals, research organizations can enhance the efficacy and integrity of their studies, leading to improved healthcare for all.




