Introduction
Clinical trials play a crucial role in medical research, evaluating the safety and effectiveness of new treatments and medical devices. They offer hope to patients seeking innovative solutions for their health conditions.
However, participating in clinical trials can present challenges, particularly when patients have to navigate logistical complexities beyond their geographical reach. In this article, we will explore the importance of clinical trials, the role of consulting firms in optimizing trial processes, marketing strategies for patient engagement, and lessons learned from past trials. Join us as we delve into the world of clinical trials and the impact they have on medical research.
The Importance of Clinical Trials in Medical Research
Clinical trials are the cornerstone of medical innovation, meticulously designed to assess the safety and efficacy of novel treatments, drugs, or medical devices. These trials represent hope for patients, like the one from rural Pennsylvania battling an ultra-rare disease without an FDA-approved cure. Offered a chance to join a clinical trial in Turkey, the patient faces not just the disease but also the daunting challenge of international travel logistics, from securing visas to navigating foreign paperwork.
This scenario underscores the complexities patients encounter when participating in life-altering research beyond their geographic reach. The landscape of finding and enrolling in clinical trials has evolved significantly. A decade ago, sifting through the National Institute of Health's website was the primary means of finding a trial, a task both overwhelming and perplexing.
Now, with the advent of online platforms like Let's Win, in collaboration with EmergingMed, patients can easily find trials tailored to their specific condition and location. However, while the treatment within a clinical trial is generally provided at no additional cost, patients must be cognizant of ancillary expenses, such as travel, additional tests, and potential loss of income from work. "Research is really the foundation for medicine," as articulated in a special communication in JAMA.
Clinical trials, driven by patient volunteers, compare new medical ideas to existing treatments, fostering incremental improvements in standard care and enhancing patient outcomes. This process is meticulously carried out with the physician, patient, and community's perspective in mind, granting patients access to therapies that may surpass the efficacy of established treatments. Nonetheless, challenges persist, as highlighted by Derek Angus and colleagues in JAMA, where the design and execution of RCTs may not always translate seamlessly into clinical practice, emphasizing the need for better integration of trials with clinical care to maximize their impact and scope.
The Role of Consulting in Clinical Trials
Navigating the complexities of clinical trials, clinical trial consulting firms offer indispensable expertise to ensure that each phase of the trial is meticulously planned and executed. These firms address a myriad of challenges, from study design and protocol development to regulatory compliance and data management.
Their role is pivotal in optimizing decision-making processes, as underscored by a case where companies admitted retrospectively that their Phase I-III studies could have benefited from more thorough planning. Examination of over 1,200 data points revealed that, in about 80% of instances, earlier and more rigorous advisory input could have significantly improved outcomes.
Furthermore, consulting services extend beyond the technical aspects, as they also tackle the logistical hurdles for participants, such as those faced by a patient from rural Pennsylvania required to travel to Turkey for a trial. The patient's ordeal of managing international travel and navigating unfamiliar bureaucratic procedures highlights the need for comprehensive support systems. By providing end-to-end solutions, consulting firms like CMIC Group, Japan's trailblazer in the CRO industry, demonstrate their capacity to meet the diverse needs of stakeholders across the entire pharmaceutical value chain.
Marketing Strategies for Clinical Trials
In the landscape of clinical trial marketing, the emergence of digital transformation and artificial intelligence (AI) has revolutionized the way companies approach patient engagement. The modern pharmaceutical consumer, accustomed to the personalized services of tech giants like Amazon and Netflix, now expects a similar experience in healthcare. This paradigm shift necessitates a customer-first marketing strategy, leveraging tools such as Machine Learning and Propensity Modeling to tailor online experiences to individual needs.
For example, consider the case of a patient from rural Pennsylvania with an ultra-rare disease and no FDA-approved treatment options. Presented with the chance to join a clinical trial in Turkey, the patient faces the daunting challenge of navigating international travel logistics, from securing visas to dealing with unfamiliar paperwork. This scenario underscores the importance of clear communication and support in clinical trial marketing efforts, ensuring that potential participants are well-informed and assisted through every step of the process.
The integration of AI and digital tools into content marketing not only enhances the user experience but also facilitates the conveyance of vital information about clinical trials. It is imperative that companies clearly articulate the trial's objectives, benefits, and potential risks to build trust and foster participation. As Bridget Seay of epocrates emphasizes, understanding the nuances between consumer and medical marketing is crucial for crafting messages that resonate with both patients and healthcare professionals, ultimately leading to successful trial outcomes.
Case Study: Effective Consulting in a Clinical Trial
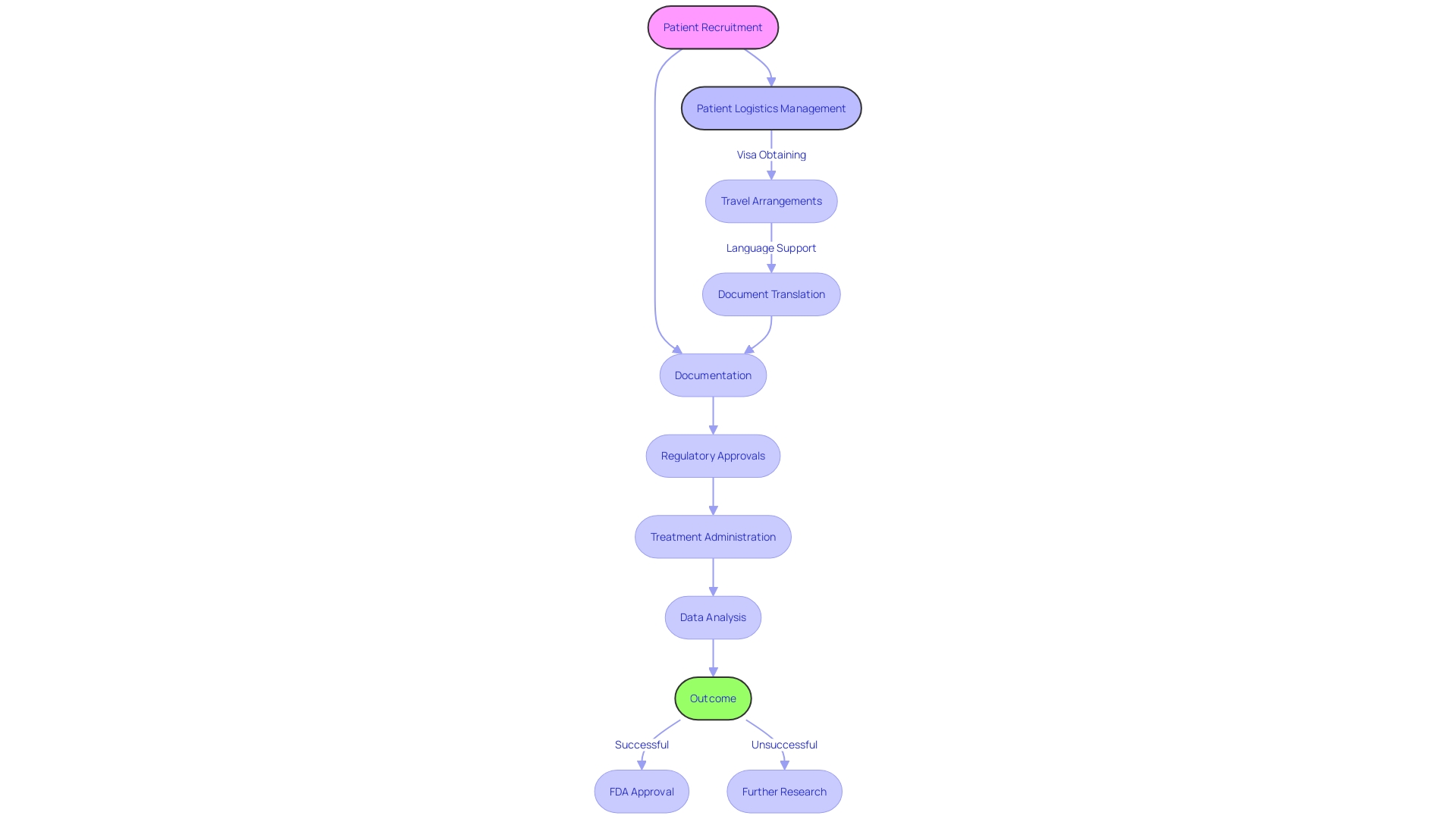
Navigating the complexities of clinical trials can be daunting, especially when they involve cross-border challenges. A poignant example is the plight of a rural Pennsylvania patient with a rare disease, contemplating participation in a life-saving trial in Turkey.
The myriad of logistical concerns, from visa procurement to language barriers in documentation, underscores the critical role of Contract Research Organizations (CROs) in facilitating patient access to innovative treatments. COMIC Group, Japan's pioneering CRO, exemplifies the expansive role these organizations play.
With over three decades of experience, COMIC provides end-to-end solutions that span the entire pharmaceutical value-chain. Their services, which include contract development, manufacturing, healthcare solutions, and market entry strategies, are tailored to meet the intricate needs of various stakeholders, from pharmaceutical companies to medical institutions.
According to industry experts, the foresight and meticulous planning by CROss can significantly influence the outcome of clinical studies. As one expert from Treehill Partners observed, many companies acknowledge in hindsight the potential to optimize their clinical trials. Approximately 80% of pivotal decisions could be enhanced by in-depth advisory services, ensuring each 'link in the chain' is fortified for the company's critical timeframes. This holistic approach by CROs like CMIC not only accelerates drug development but also bridges the gap between patients and access to cutting-edge medical research.
Lessons Learned and Best Practices
Clinical trial consulting firms, with their extensive experience in overseeing a myriad of clinical studies, have honed their expertise in enhancing trial effectiveness and efficiency. From the early stages of drug development to the critical post-study analysis, these firms apply a strategic approach to optimize each phase of the process.
An analysis of past trials reveals that approximately 80% of decisions, if more meticulously evaluated and fortified, could substantially improve future outcomes. Such insights are invaluable, particularly when considering the complex logistics faced by trial participants, such as those navigating international travel for life-saving treatments.
For instance, a patient from rural Pennsylvania may have to manage visa applications, unfamiliar paperwork, and travel coordination to participate in a trial in Turkey, underscoring the need for seamless planning and support. Moreover, Contract Research Organizations (CROs) like CMIC Group, which pioneered the CRO business in Japan, offer comprehensive services that span the entire pharmaceutical value chain. These organizations pride themselves on delivering precisely what clients need to advance their products, reflecting a commitment to innovation and customer-centric solutions. By integrating industry best practices and continually educating themselves, clinical trial companies are pivotal in streamlining drug development programs and bringing new therapies to market more effectively.
Conclusion
In conclusion, clinical trials are essential for evaluating new treatments and medical devices, providing hope to patients seeking innovative solutions. However, logistical challenges can arise when patients need to participate in trials beyond their geographical reach. Online platforms like Let's Win and EmergingMed have simplified the process of finding and enrolling in clinical trials.
Consulting firms play a crucial role in optimizing trial processes and providing comprehensive support systems for participants facing logistical hurdles. Digital transformation and AI have revolutionized marketing strategies for clinical trials, enabling personalized experiences tailored to individual needs. Clear communication and support are vital for conveying information and fostering patient participation.
Contract Research Organizations (CROs) like CMIC Group facilitate patient access to innovative treatments with their end-to-end solutions spanning the pharmaceutical value chain. Their expertise significantly influences trial outcomes. Lessons learned from past trials emphasize the importance of strategic planning and support systems.
Clinical trial consulting firms offer valuable insights to enhance trial effectiveness and efficiency. In summary, clinical trials drive medical research progress and improve patient outcomes. With the help of consulting firms, effective marketing strategies, and the integration of best practices by CROs, these trials can overcome logistical challenges and provide hope to patients worldwide.
Find and enroll in clinical trials with ease using our innovative platform.




