Introduction
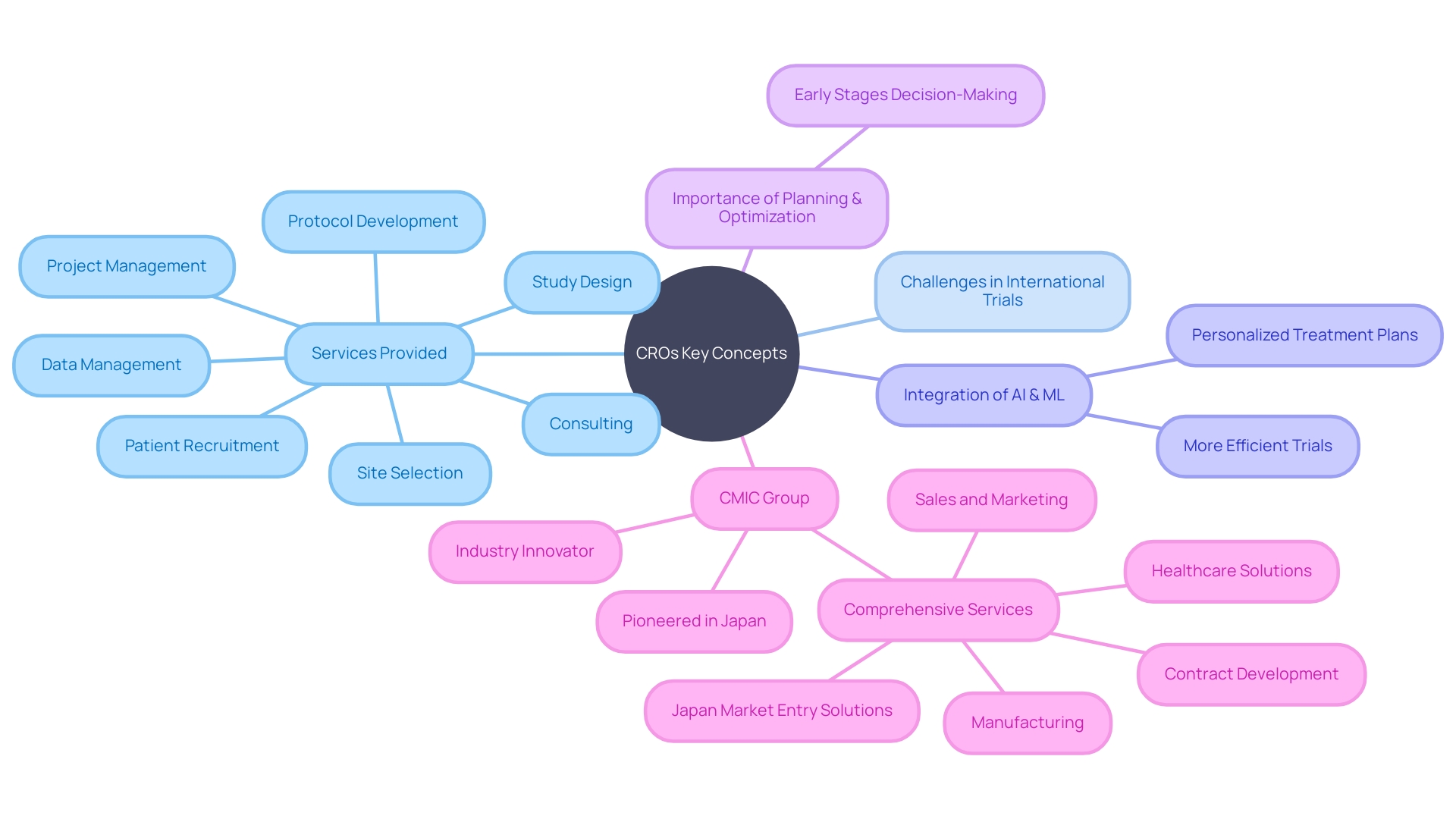
Clinical Research Organizations (CROs) play a crucial role in the field of medical research, providing comprehensive consulting services to navigate the complex process of clinical trials. From study design to project management, patient recruitment to data management, CROs offer expertise and support to pharmaceutical companies, biotech startups, and academic circles.
In addition, CROs are at the forefront of harnessing the power of artificial intelligence (AI) and machine learning (ML) to revolutionize healthcare. This article will explore the benefits of CRO consulting, its impact on patient recruitment and retention, its role in enhancing clinical trial efficiency and cost-effectiveness, successful case studies, best practices for effective CRO consulting, and the future of CRO consulting in advancing medical research.
The Role of CRO Consulting in Clinical Trials
Clinical Research Organizations (CROs) are at the forefront of transforming medical research with their comprehensive consulting services. They assist entities ranging from pharmaceutical giants to biotech startups and academic circles in navigating the complex process of clinical trials.
Their expertise is not limited to the basics of study design and protocol development; it extends to tackling the intricate challenges of project management, site selection, patient recruitment, and data management. In particular, CROss are instrumental in addressing the multifaceted issues that arise with international trials.
For instance, consider a patient from rural Pennsylvania with a rare disease and no FDA-approved treatment options, who must travel to Turkey for a clinical trial. The CROss step in to alleviate the logistical nightmare of cross-border travel, ensuring that the patient's focus remains on their health, not on the daunting tasks of obtaining visas, navigating foreign paperwork, or coordinating travel arrangements.
Moreover, CROs are harnessing the transformative power of artificial intelligence (AI) and machine learning (ML) to revolutionize the healthcare industry. With the rise of digital workflows and data storage, AI and ML are paving the way for personalized treatment plans and more efficient clinical trials. As we accumulate vast amounts of health data, the vision of a self-driving clinical trial becomes increasingly attainable, promising to enhance pharmacology, medical organizations, and patient outcomes. The integration of these technologies by CROss is not just about starting; it's about ensuring precision and accuracy in every facet of clinical research, from initial planning to final execution. As Andrew Phillips from Treehill Partners points out, reflecting on 20 years of transaction advisory, the key to successful clinical trials often lies in the early stages of decision-making, emphasizing the importance of meticulous planning and optimization at every step of the clinical trial process.
Benefits of CRO Consulting for Pharmaceutical Companies
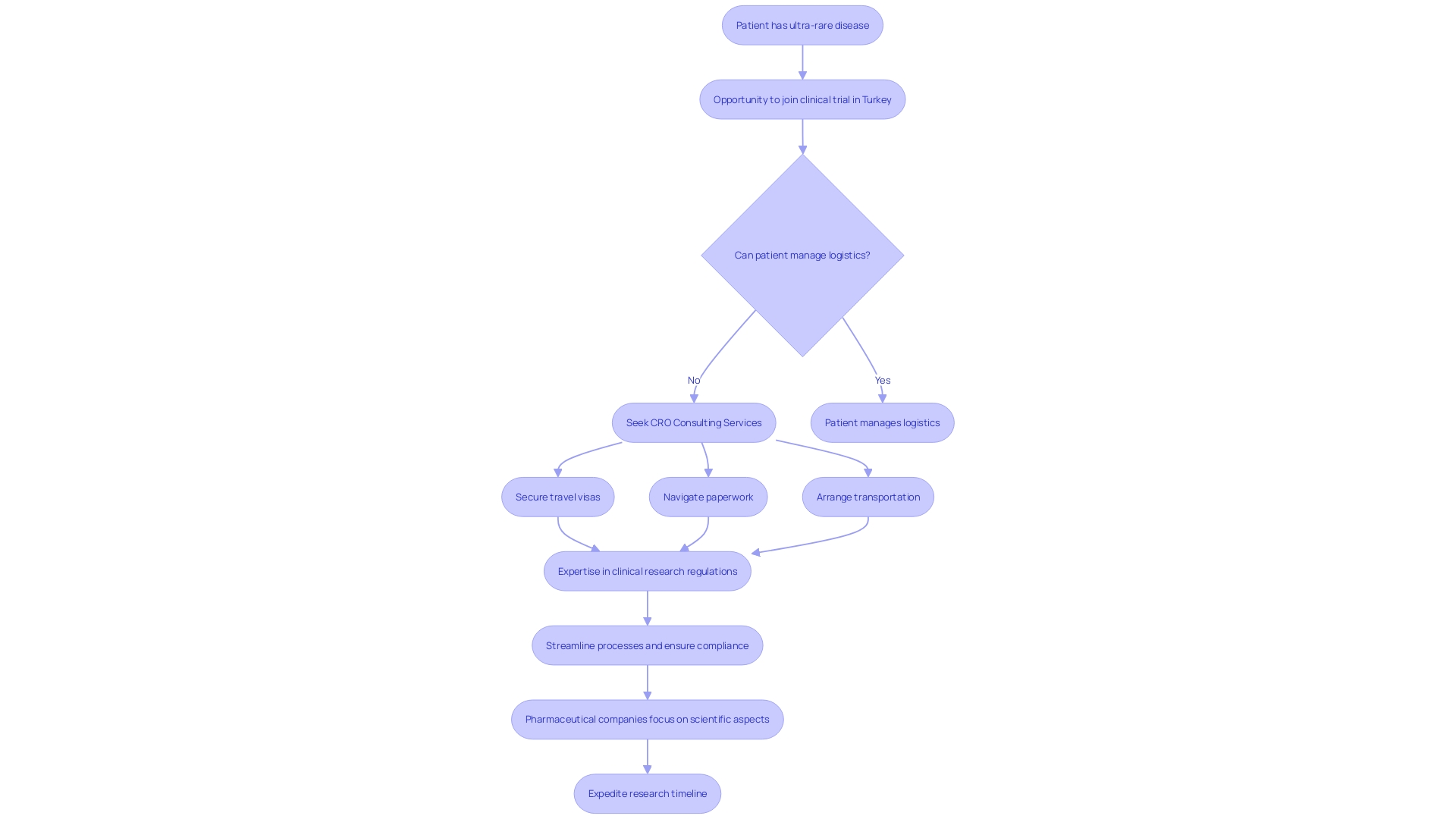
CRO consulting services are indispensable to pharmaceutical companies orchestrating clinical trials, especially when facing complex logistical challenges. For instance, imagine a patient from rural Pennsylvania with a rare condition that lacks FDA-approved treatments.
They are presented with a chance to join a clinical trial in Turkey that could potentially save their life. However, this introduces a myriad of logistical hurdles, such as securing travel visas, navigating paperwork in an unfamiliar language, and arranging transportation.
CRO consultants possess a wealth of knowledge in clinical research, including the intricacies of regulations and best practices, which can be leveraged to streamline these processes. Their expertise ensures that trials are not only run in compliance with regulatory demands but also addresses the practical aspects of trial participation for patients, such as site selection and patient recruitment. By entrusting these operational details to CROs, pharmaceutical companies can focus on the scientific aspects of the trial while minimizing the administrative burden and expediting the research timeline.
How CRO Consulting Enhances Patient Recruitment and Retention
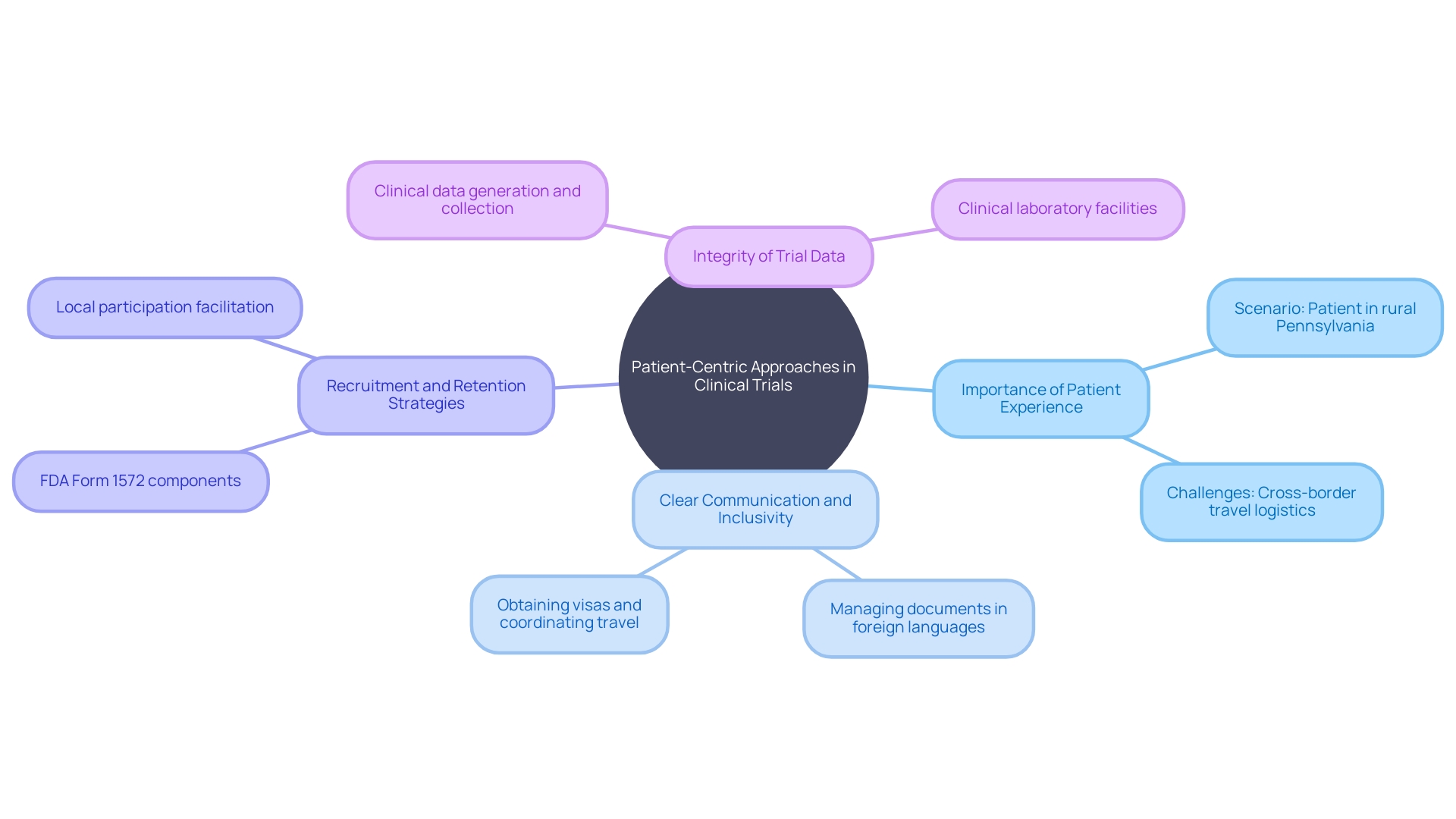
Clinical trial companies are tasked with the complex challenge of ensuring diverse and equitable patient recruitment and retention. Consider the scenario of a patient from rural Pennsylvania with an ultra-rare disease.
They're given the chance to join a clinical trial in Turkey, but the logistical hurdles of international travel, like obtaining visas and navigating paperwork in a foreign language, can be daunting. This highlights the importance of patient-centric approaches in clinical research.
As Daniel J Herron, Vice President of Digital Offerings at RWS, articulates, patient-centricity is about prioritizing the patient experience, which involves 'actively involving patients in the planning and design of trials, so their perspectives and needs are considered.' Clear communication and inclusivity are paramount, as they not only address patients' concerns but also ensure that the trial's demographic reflects a wide array of lived experiences and backgrounds. Clinical trial companies leverage this approach by using their extensive databases and networks to efficiently recruit suitable candidates and implement strategies that resonate with patients' needs, thereby enhancing retention and ensuring the integrity of trial data.
The Impact of CRO Consulting on Clinical Trial Efficiency and Cost-Effectiveness
The integration of CRO consulting into the fabric of clinical trials represents a transformative leap in advancing patient-centric care, particularly for those facing formidable challenges. Imagine a patient in rural Pennsylvania, grappling with an ultra-rare disease and devoid of FDA-approved treatments, who is offered a lifeline through a clinical trial in Turkey. The logistical hurdles of international travel, from visa procurement to navigating foreign documentation, become daunting obstacles.
CRO consultants, with their deep regulatory acumen, play a pivotal role in mitigating such complexities, enabling patients to transcend geographic barriers and access potentially life-altering therapies. Moreover, CRO consulting is at the forefront of harnessing the disruptive potential of AI and ML technologies in healthcare. The vision of a self-driving clinical trial, once a futuristic concept, is now within our grasp, driven by digital workflows and sophisticated data management.
These technologies promise a new era of personalized treatment and heightened trial efficiency, serving as a beacon for pharmacological innovation and the enhancement of patient outcomes. As we stand on the cusp of this technological renaissance, the question remains: How can we navigate this new terrain to ensure the utmost precision and efficacy in clinical trials? The answer lies in the strategic deployment of CRO consulting expertise, which is essential for navigating the evolving landscape of clinical research.
Case Studies: Successful Implementations of CRO Consulting in Medical Research
Several case studies demonstrate the successful implementations of CRO consulting in medical research. These studies highlight the positive impact of CRO consulting on various aspects of clinical trials, including study design, patient recruitment, data management, and overall trial efficiency.
For example, a case study conducted by a pharmaceutical company showed that engaging a CRO consulting service resulted in a significant reduction in patient recruitment time and increased the number of eligible patients enrolled in the trial. Another case study demonstrated how CRO consulting improved data management processes, ensuring the accuracy and integrity of trial data. These case studies provide concrete evidence of the value and effectiveness of CRO consulting in advancing medical research.
Best Practices for Effective CRO Consulting in Medical Research
Effective consultation with Contract Research Organizations (CROs) is pivotal in advancing medical research, particularly when navigating the complex regulatory landscape governed by entities such as the U.S. Food and Drug Administration (FDA). A foundational aspect of successful CRO engagement is the establishment of transparent and ongoing communication with the pharmaceutical company. This involves aligning on shared objectives, delineating precise roles and responsibilities, and fostering continuous dialogue to facilitate the clinical trial process.
Selecting a CRO with a demonstrated proficiency in the relevant therapeutic domain is equally vital, as it brings specialized knowledge and experience to the table. It is imperative for the chosen CRO to possess stringent quality management systems that uphold data integrity and adhere to regulatory standards, thereby safeguarding public health. Furthermore, the importance of consistent monitoring and assessment of the CRO's performance cannot be overstated.
This scrutiny is essential for pinpointing improvement opportunities and guaranteeing the trial's success. In the context of patient-centricity, consider the challenges faced by a patient in rural Pennsylvania with a rare disease, necessitating participation in a trial abroad. Such scenarios underscore the need for CROss to not only manage the scientific and regulatory facets of trials but also to address the logistical and human elements that impact patient participation and trial outcomes.
The Future of CRO Consulting in Advancing Medical Research
As clinical trials become increasingly global and intricate, the role of CRO consulting is expanding. Consider patients with rare diseases who may find hope in trials abroad, such as a patient in rural Pennsylvania needing to navigate the complexities of joining a study in Turkey. The logistical challenges—from securing visas to overcoming language barriers—underscore the need for CROss to provide more than just scientific expertise; they must also offer comprehensive logistical support.
Meanwhile, the integration of AI and ML in healthcare is revolutionizing clinical trials. The digitalization of workflows and data management is paving the way for more personalized treatments and efficient trial processes. The concept of a self-driving clinical trial is not far off, promising to enhance pharmacology and patient outcomes significantly.
However, translating such technological advancements to clinical practice remains a challenge. As noted in academic research, creating clinical decision tools that are widely adopted is complex, and even well-cited classification schemes for diseases like colon, breast, and pancreatic cancer often go unused in clinical settings. This gap highlights the critical role CROss will play in not just advancing medical research, but in ensuring these innovations are effectively implemented to truly benefit patient care.
Conclusion
In conclusion, Clinical Research Organizations (CROs) play a vital role in advancing medical research by providing comprehensive consulting services for clinical trials. They assist pharmaceutical companies, biotech startups, and academic circles in study design, project management, patient recruitment, and data management. CRO consulting offers numerous benefits, streamlining complex logistical challenges and allowing companies to focus on the scientific aspects of trials.
Through patient-centric approaches, CROs enhance patient recruitment and retention by involving patients in trial planning and implementing strategies that address their needs. The integration of CRO consulting improves trial efficiency and cost-effectiveness while advancing patient-centric care. CRO consultants navigate international travel barriers and leverage AI/ML technologies to personalize treatments and optimize trial processes.
Successful case studies demonstrate the value of CRO consulting in medical research, showcasing improved patient recruitment time, increased enrollment numbers, enhanced data management processes, and overall trial efficiency. To ensure effective CRO consulting, transparent communication with pharmaceutical companies is crucial. Establishing shared objectives and selecting specialized CROs with stringent quality management systems contribute to success.
As clinical trials become more global and intricate, the future of CRO consulting expands. Comprehensive logistical support alongside scientific expertise becomes essential for patients participating in trials abroad or facing complex processes like securing visas. Effective utilization of AI/ML technologies supported by CROs will be critical for personalized treatments.
In summary, CRO consulting plays a critical role in advancing medical research through expertise in navigating complex clinical trial processes. Their impact extends to patient recruitment and retention as well as improving trial efficiency and cost-effectiveness. With the integration of AI/ML technologies on the horizon, effective utilization of CRO consulting will be essential for precision in clinical research while benefiting patient care.




