Introduction
Clinical Research Organizations (CROs) play a vital role in advancing medical science by managing and conducting clinical trials for the pharmaceutical, biotechnology, and medical device sectors. Their primary objective is to ensure the efficiency, safety, and adherence to regulatory standards in these critical studies. With the complexity of managing clinical trials growing significantly, CROs are at the forefront of navigating logistical challenges, integrating new technologies, and addressing the disconnect between trials and real-world practice.
They strive to ensure that studies not only contribute to scientific knowledge but also seamlessly integrate with clinical practice. In this article, we will explore the role of CROs in the research process, the services they offer, their impact on biopharmaceutical development, data management, statistical analysis, and regulatory submissions. We will also discuss the benefits of partnering with CROs, career opportunities in clinical research, successful collaborations, and future trends and advancements in CRO research.
Join us as we delve into the transformative world of clinical research organizations.
What are Clinical Research Organizations (CROs)?
Clinical Research Organizations (CROs) serve a pivotal function in advancing medical science by managing and conducting clinical trials for the pharmaceutical, biotechnology, and medical device sectors. CROss ensure that these critical studies are executed with the utmost efficiency, safety, and adherence to the stringent regulatory standards. Collaborating with a multitude of stakeholders, including sponsors, investigators, and regulatory bodies, CROss facilitate the meticulous research process and optimize the integrity of the data procured.
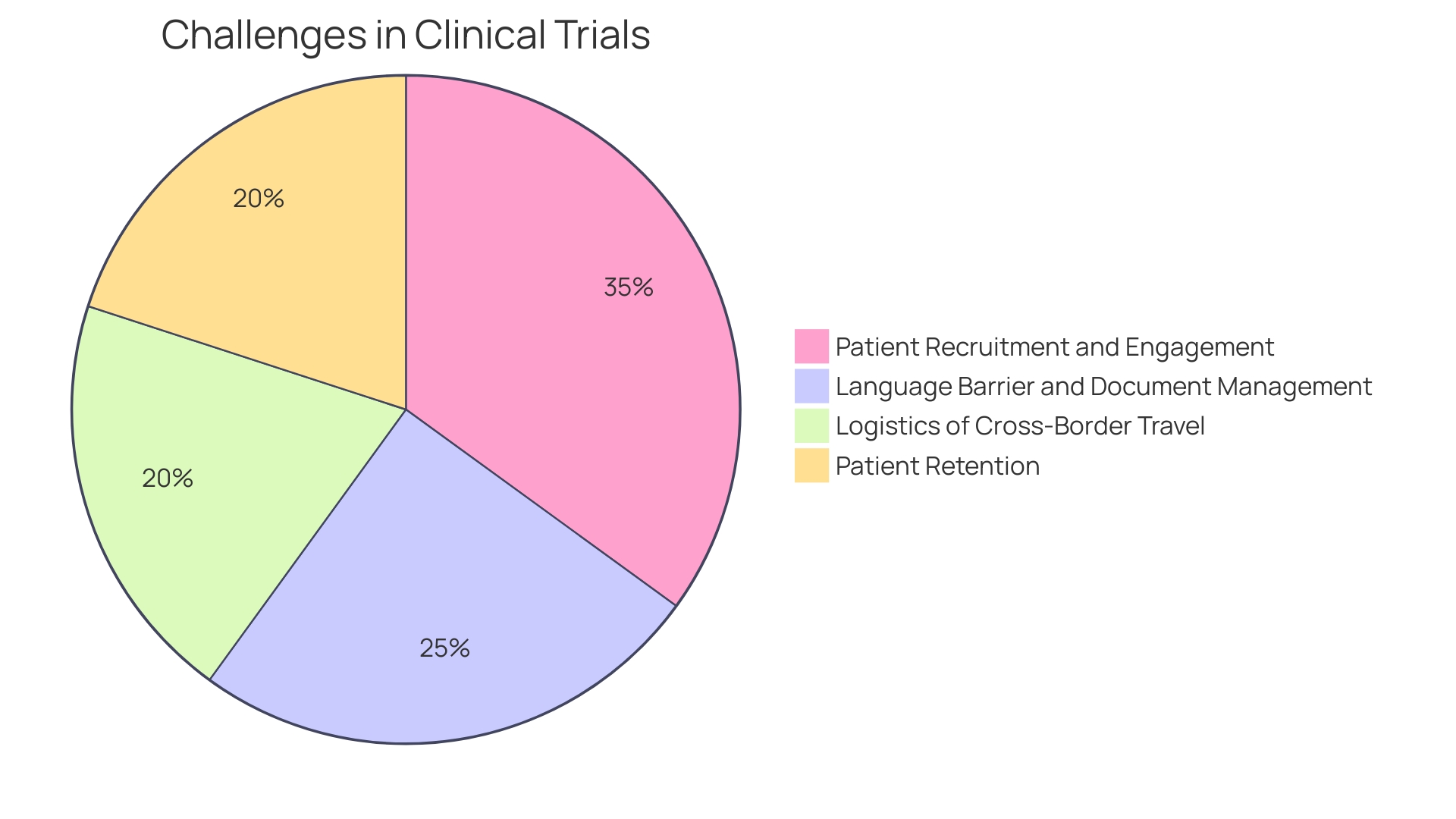
The complexity of managing clinical trials has grown significantly, considering the global scale and ethical, legal, and social implications. For instance, patients from remote areas may be presented with life-saving trial opportunities abroad, necessitating intricate logistics like cross-border travel and handling documentation in unfamiliar languages. Furthermore, the evolution of clinical trial design, propelled by technological advancements and market incentives, has highlighted the importance of balancing scientific rigor with operational efficiency.
Ken Getz, an industry expert, emphasizes this evolution, stating, "There's been growing recognition in the importance of balancing great science with great execution."
Statistics underscore the pressing need for transparency and data sharing, with influential trials nearly always registered in databases like ClinicalTrials.gov. Yet, detailed protocols and raw data remain less accessible, and industry-sponsored trials are often closely analyzed by internal statisticians without broader dissemination.
Moreover, a study highlighted in JAMA addresses the disconnect between clinical trials and practice, acknowledging the inefficiencies and scope limitations due to the siloed nature of traditional trial designs. As the industry progresses, the integration of clinical trials into practice becomes essential, with approximately 40,000 RCTs registered annually, and yet clinical guidelines often rely on expert opinion rather than robust trial evidence.
CROs are at the forefront of this transformative phase in clinical research, striving to ensure that studies not only contribute to scientific knowledge but also reflect and integrate seamlessly with real-world clinical practice.
The Role of CROs in the Research Process
Clinical research organizations (CROs) serve as pivotal partners in the realm of clinical trials, engaging in a symbiotic collaboration with sponsors to facilitate various stages of the research process. Their role encompasses the intricate planning of study designs and protocol development, as well as performing comprehensive feasibility assessments to gauge the viability of potential clinical studies. A case in point is the experience of Chris, a biomedical engineer with extensive expertise in managing clinical studies for medical devices, whose role at Greenlight Guru involves demonstrating high-quality endpoints and leveraging AI platforms to expedite imaging processing and analysis.
Moreover, CROs are instrumental in selecting appropriate sites and recruiting suitable patients, all while upholding the ethical standards and regulatory frameworks that govern clinical trials. Their involvement extends to meticulous project management, vigilant monitoring, and proficient data management, culminating in robust statistical analyses that drive the successful completion of clinical studies. The collective expertise and resources of CROss significantly enhance the execution of clinical trials, ensuring scientific accuracy and contributing to the generation of high-quality data that supports clinical development goals.
In the dynamic landscape of healthcare, where the digital therapeutics revolution is gaining momentum, CROss are increasingly adapting to the evolving needs of the industry. The rise of personalized, low-risk approaches like apps and virtual reality for treating various conditions underscores the importance of CROss in integrating new technologies into clinical research. As noted in a recent study, the clinical trial market is projected to reach $13 billion by 2026, reflecting the growing significance of these organizations in the advancement of medical treatments.
The transparency of clinical trial results remains a critical issue, as highlighted by a study reviewing 6,720 clinical trials in Canada, which revealed that only 59 percent were registered before participant enrollment and a staggering 32 percent never reported their results. This underscores the need for CROs to ensure that trial information and outcomes are readily accessible to the public, thereby enhancing the credibility and reproducibility of the research conducted.
CROs are at the forefront of incorporating AI and ML into clinical trials, striving for the highest levels of client care and data accuracy. By capitalizing on the wealth of data from connected devices and decentralized trial solutions, CROss are poised to revolutionize drug development, making it more patient-centric and data-driven. With a keen focus on data strategy, CROs are setting the stage for a future where clinical trials are more efficient and yield more precise insights, ultimately improving patient outcomes.
Services Offered by CROs
Clinical trial companies are at the forefront of medical advancements, offering a vast suite of services that extend from the initial phases of drug development to post-market analysis. These services encompass protocol development, site management, and patient engagement, aimed at ensuring the integrity and efficacy of clinical trials. Employing a diverse team of specialists—ranging from clinical research associates to data analysts and regulatory experts—these organizations are dedicated to the meticulous orchestration of clinical trials.
Contract Research Organizations (CROs) like CMIC Group, which initiated the CRO model in Japan, have evolved to offer comprehensive solutions across the entire pharmaceutical value chain. Their innovative services cater to pharmaceutical entities, medical institutions, and academia, among others, ensuring that each stakeholder receives precisely what is needed to propel their products and research forward.
Innovative trial designs, such as the R-RCT model utilized in the DAPA-MI study, blend the authenticity of real-world data with the methodological rigor of randomized trials. This approach allows for the inclusion of a broader patient population, offering a cost-effective alternative while maintaining the robustness required for regulatory approval.
Clinical trials are critical for the development of new medical interventions, and their execution must adhere to the highest standards. The phases of clinical trials, from safety-focused Phase one to efficacy-assessing Phase two and beyond, are meticulously planned to ensure patient safety and reliable outcomes.
Despite the progress, clinical trials face significant hurdles, such as patient recruitment and retention, with nearly 80% of trials not finishing on schedule. This not only has financial repercussions but can also delay the availability of potentially life-saving treatments for patients.
The narrative of a Pennsylvania resident with an ultra-rare disease considering participation in a trial abroad underscores the logistical and linguistic challenges faced by patients and their families. These personal stories highlight the need for more accessible clinical trials and the importance of ensuring a positive experience for participants.
CROs, by leveraging their expertise, strive to mitigate these challenges and enhance the quality of clinical trials. Their work is essential to the discovery of new treatments that can lead to improved patient outcomes, healthier populations, and more efficient healthcare systems. As the landscape of clinical research evolves, CROss continue to play a pivotal role in shaping the future of healthcare.
Biopharmaceutical Development and Clinical Trials Management
Contract Research Organizations (CROs) are pivotal in advancing the biopharmaceutical industry, offering comprehensive support from the earliest stages of drug development through to the final phases of clinical trials. They bring critical expertise to the table in early-phase studies, especially first-in-human trials that ascertain the safety profile of new investigational drugs. As the drug development journey progresses, CROss are instrumental in orchestrating Phase II and Phase III trials, which are larger and more complex, focusing on the drug's efficacy and safety in broader patient populations.
The role of CROs extends beyond mere facilitation; they are strategic partners in managing the multifaceted aspects of clinical trials, including robust data collection and sophisticated analysis. This partnership is crucial, as insights from clinical trials pave the way for transformative healthcare solutions that could potentially change lives. For instance, the utilization of advanced technologies such as AI is revolutionizing drug development, as evidenced by discussions at industry conferences like Bioworld's annual Biofuture event.
AI is forecasted to expedite the identification of effective molecules and substantially reduce clinical trial durations.
Moreover, the strategic input of CROs can have a profound impact on the outcome of clinical trials. As highlighted by industry advisors, 80% of decisions made during the drug development process could be optimized if more time and energy were dedicated to scrutinizing these critical choices. CROs can potentially enhance the effectiveness of each decision, thereby exponentially improving subsequent phases of the trial.
This comprehensive approach could address the common issue where a staggering 80% of clinical trials do not conclude on schedule, underscoring the importance of meticulous planning and execution in clinical trial management.
CROs' contributions are vital in a healthcare landscape marked by competitive pressures, such as the need to be the first to market with novel treatments, and the urgency to meet the needs of patients with unmet medical conditions. Their expertise is not just about meeting regulatory approval criteria; it's about envisioning the future of healthcare and actively shaping it through strategic, data-driven decisions that can lead to significant medical breakthroughs and the delivery of life-saving therapies to patients worldwide.
Data Management, Statistical Analysis, and Regulatory Submissions
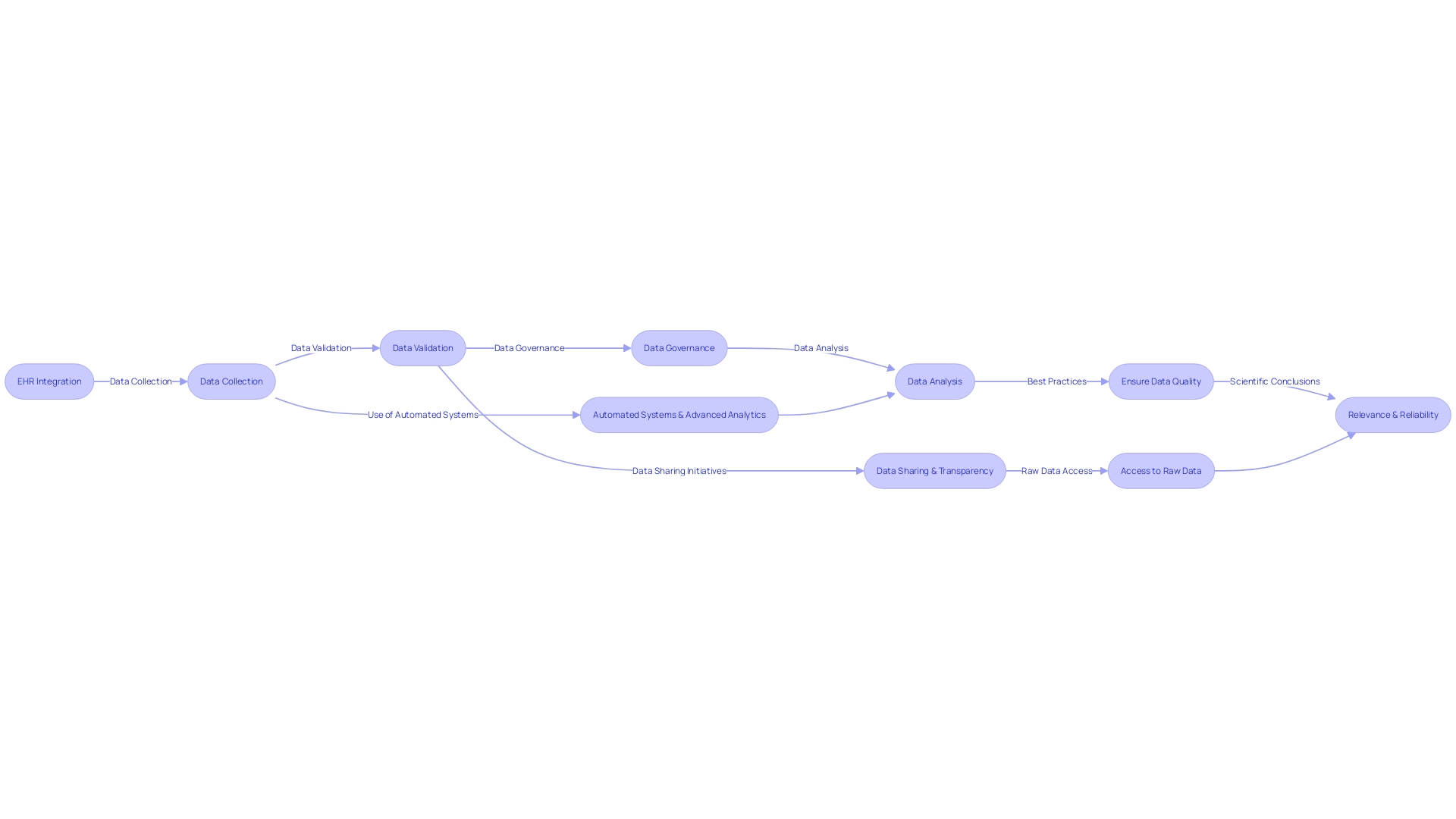
Clinical trial companies, also known as Contract Research Organizations (CROs), are pivotal in harnessing the power of data to drive medical breakthroughs. These organizations expertly manage the voluminous and complex data generated during clinical trials, ensuring its quality, integrity, and confidentiality. With the advent of electronic health records (EHRs) and the integration of various data sources, the role of CROss has become even more critical.
For instance, a study highlighted the implementation of EHR data to complement traditional data collection methods in a multi-center pharmaceutical trial, emphasizing the need for robust data management systems (Raman et al., Trials 2023, 24:566).
The volume of data in today's clinical trials is staggering, with a single Phase 3 trial producing an average of 3.6 million data points. This marks a threefold increase from the amount of data collected a decade ago (FDA-2001-D-0219). CROss facilitate the efficient handling of this data through automated systems and advanced analytics, enabling researchers to extract valuable insights that support regulatory submissions, such as clinical study reports and Investigational New Drug (IND) applications.
Addressing data quality in clinical research is paramount, as evidenced by the scrutiny of an Alzheimer's disease study where image integrity was called into question (Proofig AI, 2022). CROs address this issue by implementing best practices for data validation and governance, with an emphasis on ensuring the relevance and reliability of the data used to inform scientific conclusions (Flatiron Health). With the growing emphasis on patient-centered drug development and the integration of real-world data, CROss are at the forefront of defining quality frameworks that harmonize different industry standards, thereby fostering trust and credibility in clinical research outcomes.
Benefits of Partnering with CROs
Clinical Research Organizations (CROs) are pivotal in the landscape of medical advancements, offering a wealth of specialized knowledge and resources. They stand as indispensable allies to sponsors and research entities, streamlining the clinical trial process with their profound experience and expertise. This partnership enables sponsors to tap into a vast network of potential study sites and glean from CROs' established rapport with regulatory authorities and investigators.
Moreover, CROs' involvement empowers sponsors to concentrate on their principal expertise, entrusting the intricacies of research management to adept professionals.
For instance, the collaboration between MedRhythms and Curavit illustrates the dynamic capabilities of CROs in managing intricate hybrid clinical trials. This partnership underscores the vital role of CROs in orchestrating clinical trials while addressing the economic implications for the healthcare system.
Furthermore, CROs are at the forefront of addressing the critical elements of patient centricity and diversity in clinical research. As Daniel J Herron emphasizes, a patient-centered approach is paramount, involving patients in trial design and ensuring comprehensible information delivery. This focus on diversity and inclusion ensures a broad representation of demographics, which is essential for comprehensive and equitable research outcomes.
The significance of CROss is also reflected in the growing trend of patient organizations seeking partnerships with life sciences companies. With an increasing number of patient organizations established, particularly those focusing on rare diseases and oncology, CROs become essential in facilitating these collaborations, bringing new treatments into the limelight, and ensuring patients are well-informed.
In summary, CROs are not merely facilitators but catalysts of innovation in clinical research, driving forward the mission of enhancing patient outcomes through efficient and effective trial management.
Career Opportunities in Clinical Research
Clinical research organizations (CROs) are at the forefront of scientific advancement and offer diverse career paths for professionals from various backgrounds such as life sciences, medicine, and pharmacy. These roles range from clinical research associates to project managers, statisticians, and regulatory specialists. A notable example is Anna Danielyan, MD, who transitioned from a decade-long career in pediatrics to a clinical scientist role at a biotech company, illustrating the dynamic career mobility within the industry.
Her journey underscores the importance of ongoing education, as evidenced by her enrollment in Harvard Medical School's Foundations of Clinical Research to enhance her research skills.
In the field of clinical research, the integration of data science is paramount. Professionals with expertise in statistics or biostatistics, exemplified by those at Bristol Myers Squibb, play a critical role in the development of new therapies across various medical disciplines. This intersection of data and life sciences is also a focal point for BioTalent Social, an event and networking platform that fosters discussions on industry trends and challenges, including diversity and employer branding.
The impact of CRO work extends beyond individual career growth, contributing to pivotal cancer research and treatment. Professor James Catto's work, which spans prevention, diagnosis, and quality of life, reflects the sector's commitment to improving patient outcomes through a multimodal approach. Such efforts are essential, as nearly 80% of clinical trials experience delays, often due to patient recruitment and retention challenges, highlighting the need for skilled professionals to navigate and mitigate these complexities.
CROs provide a collaborative environment where individuals like Dr. Jasna Markovac apply their scientific background and consulting expertise to enhance academic and medical research practices. Dr. Markovac's career trajectory, from genetics and molecular biology to influential roles in scientific publishing and academia, demonstrates the breadth of opportunities available within clinical research organizations. As the medical landscape evolves with clinical trials playing a crucial role, the demand for professionals who are well-versed in regulatory and ethical standards, and who can contribute to the collective goal of advancing medical science, continues to rise.
Case Studies: Successful Collaborations with CROs
Case studies exemplify the transformative power of partnerships between clinical trial sponsors and Contract Research Organizations (CROs) in enhancing medical science. For example, through the online participant matching portal The New Normal, researchers have been able to recruit participants, including those like Barbara, who discovered an undiagnosed heart condition through her involvement in a study, emphasizing the surprise benefits for participants in clinical trials. Her story, along with others, underscores how clinical trials can uncover critical health information that might otherwise remain unnoticed.
In another scenario, philanthropic support played a pivotal role in a research lab's ability to pursue groundbreaking work beyond its usual capacity. A poignant narrative illustrates this when a family, motivated by a personal connection to the research being conducted by Dr. Huang on prostate cancer, provided funding that was instrumental in advancing the development of novel therapies.
Moreover, embracing technology and collaboration, companies like Genentech are integrating Artificial Intelligence (AI) into the drug development process. This approach aims to streamline and predict successful outcomes more accurately, as reported by medical news outlets.
The significance of these collaborative efforts is further highlighted by statistics showing an uptick in science and technology agreements globally, demonstrating a trend towards increased cooperation in scientific endeavors. In the realm of patient advocacy, nearly 700 deals between patient organizations and life sciences companies have been documented in the last 15 years, signifying a notable partnership in advancing patient care and research.
These real-world examples and data points collectively portray the essential role of CROs in clinical trials, from providing unexpected health insights to participants to fostering research through strategic partnerships and financial support, thus driving medical advancements and patient care to new heights.
Future Trends and Advancements in CRO Research
Clinical research organizations (CROs) are at the cusp of a transformative era, with emerging technologies paving the way for a future that promises enhanced efficiency and improved patient outcomes. Advancements such as artificial intelligence (AI) and machine learning (ML) are revolutionizing data analysis, providing the tools for more personalized treatments and faster clinical trial processes. A notable development is the utilization of AI in predicting disease progression, as demonstrated by Cambridge scientists who created an AI tool that accurately predicts Alzheimer's disease progression in 80% of cases with early signs of dementia.
This innovation could potentially eliminate the need for invasive diagnostic procedures, thereby optimizing early therapeutic interventions.
Decentralized clinical trials (DCTs) are another breakthrough, addressing logistical challenges for patients in remote locations. For instance, a patient from rural Pennsylvania with a rare disease may face the daunting task of traveling to Turkey for a clinical trial. DCTs can alleviate such burdens, offering remote participation options and thereby expanding access to groundbreaking treatments.
Real-world evidence (RWE) is also gaining momentum, integrating data from actual patient experiences to inform research studies. The integration of RWE promotes a more comprehensive understanding of how treatments perform in diverse, real-life scenarios, outside the controlled conditions of traditional clinical trials.
The potential impact of these advancements on CRO research is substantial. As observed in a survey of 95 randomized controlled trials, the clinical integration of AI tools is on the rise, though with mixed results. To harness the full potential of AI/ML, it is essential to navigate the challenges of adoption, focusing on reliability, fairness, and the harmonization with clinical workflows.
The FDA's proactive approach in embracing AI for medical product evaluation, as reflected in the collaborative efforts of its various centers, illustrates the commitment to fostering innovation while ensuring public health safety. This regulatory support is crucial for the successful integration of AI into healthcare.
In summary, the future of CRO research is intrinsically linked to technological progress, with AI/ML, DCTs, and RWE at the forefront. These advancements promise to streamline the research process, elevate data quality, and expedite the development of innovative therapies, ultimately reshaping the landscape of clinical research.
Conclusion
Clinical Research Organizations (CROs) are crucial in advancing medical science by managing and conducting clinical trials. They ensure efficiency, safety, and adherence to regulatory standards while integrating new technologies and bridging the gap between trials and real-world practice. CROs play a vital role in contributing to scientific knowledge and seamlessly integrating studies with clinical practice.
CROs assist in study design, protocol development, site selection, patient recruitment, project management, monitoring, and data management. Their expertise enhances the execution of trials, ensuring scientific accuracy and generating high-quality data. CROs adapt to industry needs, incorporating technologies like AI and promoting transparency in trial results.
Offering a comprehensive suite of services, CROs provide protocol development, site management, patient engagement, and regulatory expertise. They propel products and research forward, catering to various stakeholders across the pharmaceutical value chain.
In biopharmaceutical development, CROs bring critical expertise to early-phase studies and orchestrate larger Phase II and III trials. Their strategic input and data management capabilities impact trial outcomes and contribute to transformative healthcare solutions. CROs ensure data quality, implement best practices, and harmonize industry standards for trustworthy research outcomes.
Partnering with CROs offers benefits such as access to study sites, regulatory rapport, and the ability to focus on core expertise. CROs promote patient centricity, diversity, and inclusion in research. They collaborate with patient organizations and drive medical advancements.
CROs provide diverse career opportunities in clinical research. From research associates to statisticians and regulatory specialists, CROs offer a dynamic environment for growth. Ongoing education and data science expertise are valuable in the field.
Successful collaborations between sponsors and CROs enhance medical science. They provide health insights, advance research beyond capacity, and integrate AI into drug development. These partnerships drive medical advancements and patient care.
The future of CRO research includes emerging technologies like AI, ML, decentralized trials, and real-world evidence. These advancements promise improved efficiency, patient outcomes, and reshaping the research landscape.
In conclusion, CROs are essential in advancing medical science, ensuring trial integrity, and driving healthcare solutions. Their expertise, adaptability, and commitment contribute to improved outcomes, healthier populations, and efficient healthcare systems.




