Introduction
The healthcare industry is continuously evolving with the introduction of innovative medical devices that aim to improve patient care. However, navigating the regulatory challenges associated with bringing these devices to market can be complex and time-consuming. In order to comply with FDA regulations and ensure a smooth transition from innovation to patient care, medical device companies often partner with Contract Research Organizations (CROs).
These CROs specialize in regulatory compliance and offer crucial support throughout the clinical study process. This article explores the importance of CROs in the medical device industry, the benefits of partnering with them, and provides a case study highlighting a successful collaboration between Novineon, a cardiovascular medical device company, and a specialized CRO. Stay tuned to learn more about how CROs play a pivotal role in navigating the regulatory landscape and ensuring the successful development of medical devices.
Background
The healthcare landscape is continuously transformed by innovative medical devices that promise to enhance patient care and treatment options. These devices, ranging from diagnostic tools to therapeutic interventions, are the product of extensive research and development by medical device companies.
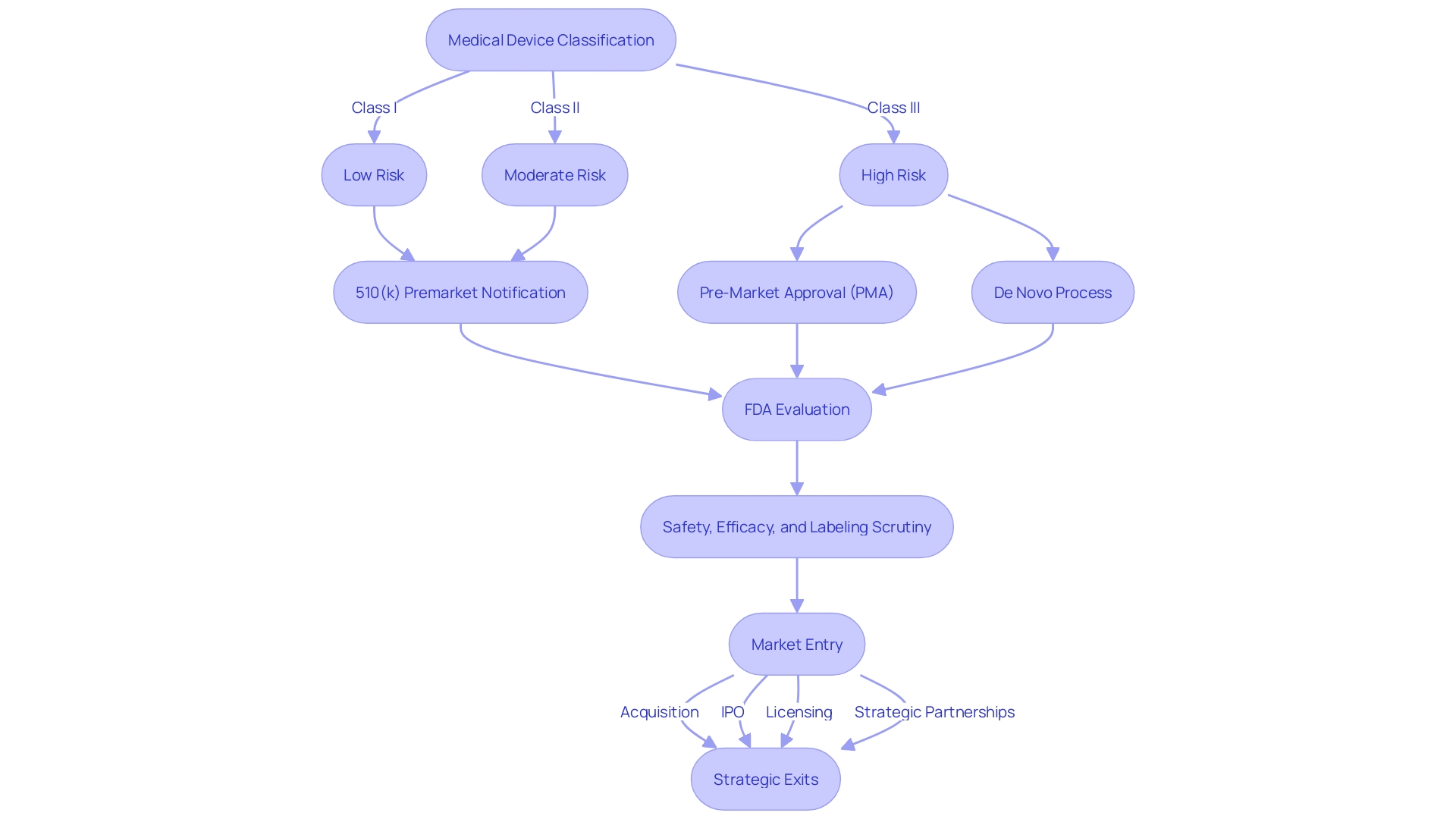
Nevertheless, the journey from concept to clinical application is fraught with regulatory challenges, especially during the critical stages of first-in-human (FIH) and early-feasibility studies (EFS). To comply with the Food and Drug Administration (FDA) regulations, a company must first accurately classify its medical device, which determines the level of risk it poses to patients.
The classification then dictates the appropriate registration pathway, be it 510(k) Premarket Notification, Pre-Market Approval (PMA), or the De Novo process. It is imperative for companies to understand the nuances between FDA terms such as Registered, Cleared, Approved, and Granted, as each represents a different stage in the regulatory process.
Navigating these complex regulatory waters is more than a mere compliance exercise; it is a strategic endeavor that can significantly impact the time and cost of bringing a medical device to market. The intricacies of regulatory compliance can introduce delays, adding to the 12 to 15 years it typically takes for a drug to traverse from research development to FDA approval. The FDA's rigorous evaluation process, conducted by an independent group of experts, scrutinizes every aspect of the medication's safety, efficacy, and labeling. The stakes are high in the medical device industry, where successful exits through acquisition, IPOs, licensing, or strategic partnerships hinge on the ability to navigate the regulatory environment effectively. The Medical Devices Industry Mergers and Acquisitions Deals report for Q3 2023 reflects the dynamic nature of the industry, highlighting the necessity for companies to stay abreast of regulatory changes to ensure a timely and profitable transition from innovation to patient care.
The Need for Medical Device CROs
In the fast-evolving medical device industry, where innovation is rapid and patient safety is paramount, regulatory oversight is intensifying. Authorities like the FDA and EMA continuously update guidelines to ensure patient safety and align with technological advancements. With an increasing number of devices in the pipeline, companies face the challenge of keeping pace with regulatory changes, which, if not managed properly, can significantly delay market entry.
To navigate this complex landscape, medical device companies often engage Contract Research Organizations (CROs). These entities bring specialized expertise in the regulatory domain, offering support that is crucial for the successful execution of clinical studies. A CRO's role becomes even more critical when considering the FDA's classification of medical devices based on risk levels.
Class three devices, for example, which include life-sustaining implants like pacemakers, require a more stringent review process and represent about 10% of FDA-regulated devices. The partnership with a CRO can be a strategic move to streamline the intricate approval process for such high-risk devices. As highlighted by industry veterans, the combination of traits like perseverance and strategic partnerships, including those with CROss, can lead to successful outcomes for medical device startups.
Whether the end goal is acquisition, IPO, licensing, or forming strategic alliances, navigating the regulatory environment effectively is a key factor in achieving a successful exit or continued operation. Medical device development companies are therefore increasingly sought after for their comprehensive services, from concept to market launch, which include a mastery of regulatory compliance and market dynamics. Stakeholders recognize that a medical device developer with a full spectrum of services can facilitate a smooth transition through development phases, ultimately saving time, money, and enhancing the success of the project.
Benefits of Partnering with a Medical Device CRO
Contract Research Organizations (CROs) are pivotal in the medical device industry, especially for navigating the intricate regulatory environments essential for first-in-human (FIH) and early-feasibility studies. With the healthcare sector undergoing rapid technological advancements, the regulatory landscape has become increasingly dynamic.
The US FDA categorizes medical devices into three classes, with the highest-risk class three devices, such as life-sustaining implantables, undergoing the most stringent regulatory scrutiny. As such, CROs' expertise in regulatory knowledge is not just about compliance but also about adapting to the frequent updates from authorities like the FDA and EMA to ensure patient safety and product efficacy.
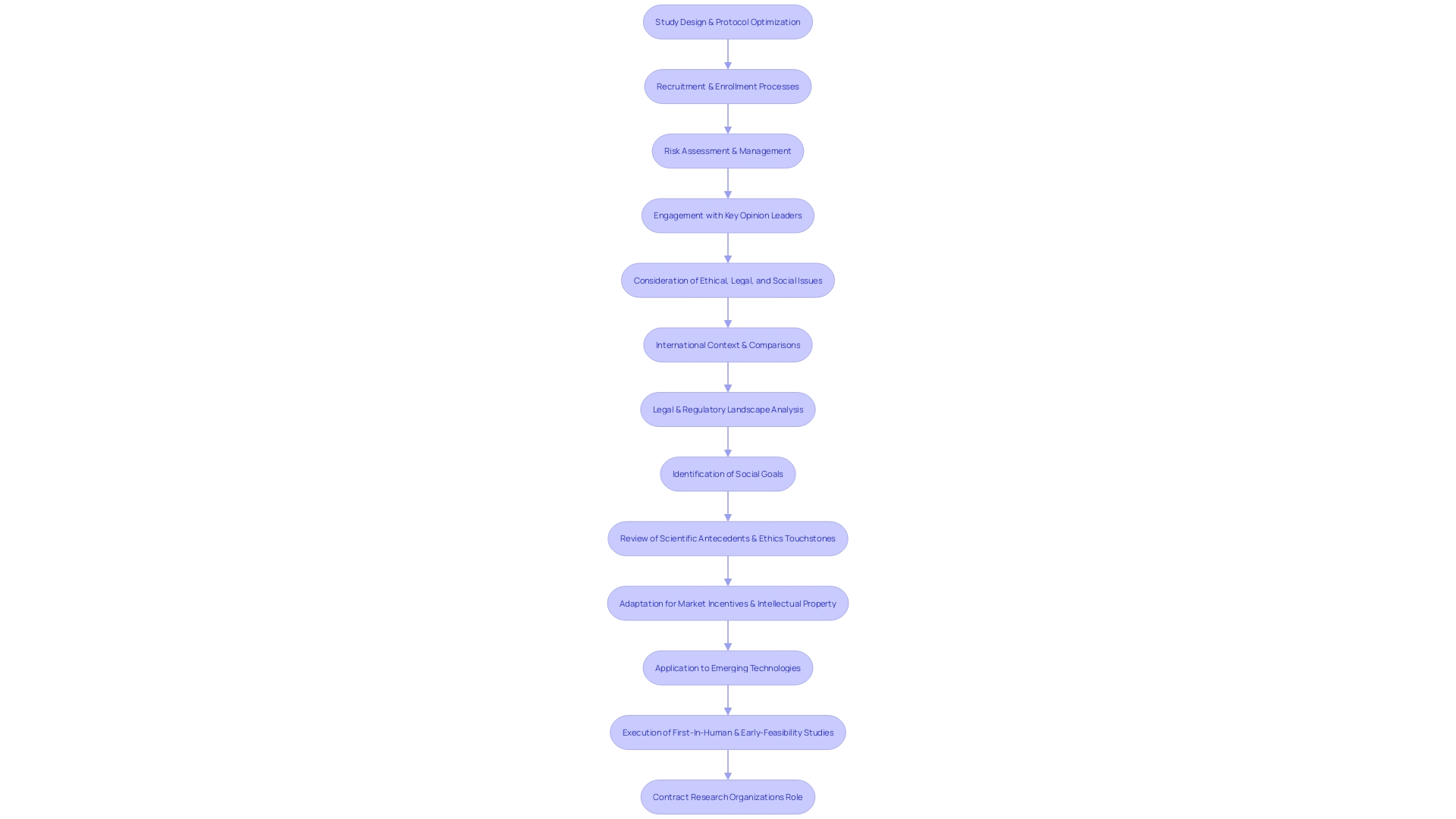
CROs are adept at efficient study design and execution, optimizing study protocols, and managing resources to avoid delays. Their experience in streamlining recruitment and enrollment processes is crucial given the evolving nature of medical devices, which are increasingly becoming digitized and complex.
This complexity is reflected in the need for a systems approach to optics in device design, where considerations span across multiple disciplines, including electrical and mechanical engineering. Furthermore, CROs maintain relationships with key opinion leaders, facilitating valuable collaborations that can enhance the study's credibility.
These networks can be vital for successful medical device exits, whether through acquisition, IPO, licensing, or partnerships, as noted by industry leaders with decades of experience. Risk mitigation is another core strength of CROs. They provide comprehensive risk assessment and management strategies, which are essential in a sector where even a minor oversight in regulatory compliance can lead to significant setbacks. For instance, the OECD's Conflict Minerals policy underscores the importance of due diligence in sourcing practices, which can impact medical device compliance. In conclusion, the role of CROs extends beyond just regulatory navigation; they are integral to the overall strategy and success of medical device clinical studies, ensuring that innovations reach the market without compromising on safety or effectiveness.
Case Study: Novineon - A Successful Collaboration
Novineon, a pioneer in the field of cardiovascular medical devices, encountered the intricate regulatory maze while aiming to conduct a first-in-human study for the cutting-edge device. Acknowledging the intricacies of regulatory compliance, Novineon sought the expertise of a specialized medical device CRO.
This partnership was instrumental in guiding Novineon through the stringent regulatory requirements, which are especially rigorous for high-risk class three devices like theirs, which represent a mere 10% of medical devices regulated by the FDA and necessitate a more complex approval process. The CRO's proficiency in study design and logistics management was pivotal in streamlining the enrollment process, ensuring adherence to regulatory standards, and facilitating the timely completion of the study. This collaboration culminated in Novineon securing regulatory approval, marking a significant milestone in the advancement of cardiovascular healthcare solutions.
Conclusion
In conclusion, the healthcare industry faces significant regulatory challenges when bringing innovative medical devices to market. Navigating these challenges is crucial for companies to ensure a smooth transition from innovation to patient care. This is where Contract Research Organizations (CROs) play a pivotal role.
CROs specialize in regulatory compliance and offer crucial support throughout the clinical study process. Partnering with a CRO brings several benefits to medical device companies. CROs have specialized expertise in navigating the complex regulatory landscape, keeping up with frequent updates from authorities like the FDA and EMA.
They optimize study protocols, manage resources efficiently, and streamline recruitment and enrollment processes. Additionally, CROs maintain valuable collaborations with key opinion leaders, enhancing the credibility of studies. Furthermore, CROs provide comprehensive risk assessment and management strategies, mitigating potential setbacks caused by minor oversights in regulatory compliance.
Their expertise extends beyond regulatory navigation; they contribute to the overall strategy and success of medical device clinical studies. A case study involving Novineon, a cardiovascular medical device company, exemplifies the successful collaboration between a company and a specialized CRO. Novineon's partnership with a CRO guided them through the stringent regulatory requirements for their high-risk class three device, streamlining the enrollment process and ensuring adherence to regulatory standards.
This collaboration ultimately resulted in Novineon securing regulatory approval for their innovative device. In summary, partnering with a CRO is essential for medical device companies to navigate the complex regulatory landscape successfully. The expertise and support provided by CROs are crucial for ensuring that innovative medical devices reach the market without compromising on safety or effectiveness.




