Introduction
Clinical trials are the backbone of medical innovation, providing crucial insights into the safety and effectiveness of new medical technologies. These trials meticulously evaluate devices, procedures, and strategies within real-world clinical settings. The importance of this process is exemplified by the challenges faced by Robin Roberts at Novartis, who needed to determine the effectiveness of digital health technologies in specific scenarios.
The stakes were high, as incorrect estimations could have significant financial implications.
The complexity of clinical trials is demonstrated by a health system's initiative to reduce inpatient mortality, which involved an innovation competition and the development of an AI tool to tackle sepsis. This intricate process showcased the complexity of medical research endeavors.
Recent news highlights the dynamic nature of the field, with promising projects entering clinical evaluation and companies like Archetype launching to address the alarming statistic that three-quarters of MedTech innovations fail to reach the market. Clinical trials also face the challenges of globalization, requiring a patient-centric approach that is both scientifically rigorous and accessible.
The volume of data in healthcare is expanding rapidly, with Phase 3 trials generating an average of 3.6 million data points. This necessitates advanced data management and analysis to streamline the trial process. Technologies such as digital patient engagement tools and wearable devices contribute to more efficient outcome assessments and data consistency.
These examples and initiatives reflect the evolving landscape of MedTech clinical trials, where innovation, data management, and patient-centric approaches are imperative for advancing healthcare and delivering life-changing medical technologies.
The Importance of Medtech Clinical Trials in Healthcare Innovation
Clinical trials are the cornerstone of medical innovation, providing essential insights that underpin the safety and efficacy of novel medical technologies. These trials meticulously evaluate medical devices, diagnostic procedures, and therapeutic strategies within real-world clinical settings. The importance of this process is highlighted by the case of Robin Roberts at Novartis, who faced challenges in determining the effectiveness of digital health technologies in specific scenarios.
The stakes were considerable, as incorrect estimations could lead to substantial financial implications.
The complexity of clinical trials is well-illustrated by a health system's initiative to reduce inpatient mortality, which involved an innovation competition and the development of an AI tool to tackle sepsis. The development lifecycle spanned from problem identification to the integration of the AI tool into clinical care, showcasing the intricate nature of such medical research endeavors.
Moreover, recent news underscores the dynamic nature of the field. A promising project is set to enter clinical evaluation with 40 adult participants next year, having gained industry support and recognition for its potential to transform patient monitoring. Similarly, Archetype's launch as a MedTech innovation management consultancy aims to address the alarming statistic that about three-quarters of MedTech innovations fail to reach the market.
By offering comprehensive services, Archetype seeks to expedite the journey of medical devices from concept to market approval.
Clinical trials also have to adapt to the challenges of globalization. A patient from rural Pennsylvania suffering from a rare disease may have to travel to Turkey for a clinical trial, navigating the complexities of international travel and language barriers. This underscores the need for a clinical trial process that is not only scientifically rigorous but also patient-centric and accessible.
The volume of data in healthcare is expanding at an unprecedented rate, with a Phase 3 trial now generating an average of 3.6 million data points. This deluge of information necessitates sophisticated data management and analysis to elevate clinical trials. Technologies such as digital patient engagement tools, wearable devices, and sensors contribute to more efficient outcome assessments and data consistency.
The integration of real-time data into centralized databases allows for immediate analysis and the identification of safety issues, streamlining the trial process significantly.
These examples and initiatives reflect the evolving landscape of Medtech clinical trials, where innovation, data management, and patient-centric approaches are imperative for advancing healthcare and delivering life-changing medical technologies to those in need.
Case Study Overview: Successful Medtech Clinical Trials
Med4Tech's innovative training program has bridged the gap between high-tech and healthcare, equipping technologists with a profound understanding of clinical processes, medical terminology, and regulatory challenges. This comprehensive education, covering a range of medical fields from emergency to radiology, is a testament to the collaboration between tech experts and healthcare professionals. It's designed to foster the creation of relevant technologies that meet the industry's demands and ultimately, improve patient care.
In a dynamic landscape where approximately 75% of MedTech innovations struggle to reach the market, companies like Archetype are emerging as pivotal players. Archetype, steered by Dr. Stuart Grant, leverages a global network of experts to navigate the intricate journey of MedTech product design, ensuring innovations efficiently achieve market approval. Dr. Grant's extensive experience in leading MedTech initiatives underscores the critical need for comprehensive strategies that address customer needs, risk management, and regulatory compliance.
The healthcare technology leader, Medtronic, with its global presence and diverse portfolio, embodies the relentless pursuit of solving complex health challenges. Its mission to alleviate pain, restore health, and extend life is brought to life through innovative technologies that impact millions worldwide every day. This organization's commitment to insight-driven care showcases the transformative power of MedTech in improving patient outcomes.
The healthcare delivery model, previously resting on a 'four-legged chair' involving patients, providers, plans, and pharmaceutical companies, has evolved. Today, digital health strategies and consumer apps have become integral to this model, reflecting a shift towards more patient-centric solutions. This evolution is further exemplified by life sciences companies integrating digital health into their offerings, bridging the gap between traditional healthcare and modern technology.
A striking illustration of the challenges faced by patients in the digital age is the story of a Pennsylvania patient with an ultra-rare disease. Offered a clinical trial in Turkey, they confronted the daunting task of navigating international travel logistics, highlighting the growing need for support systems that enable global patient participation in clinical research.
The vast increase in healthcare data, with a Phase 3 trial now generating approximately 3.6 million data points, emphasizes the importance of advanced data management and analysis in elevating clinical trials. As the volume of medical data continues to double at an unprecedented rate, the industry's capacity to harness this information becomes essential for driving successful trial outcomes and fostering innovation.
In conclusion, the MedTech sector's advancement hinges on interdisciplinary collaboration, patient-focused innovation, and strategic market navigation, underpinned by a robust understanding of the complex healthcare ecosystem.
Best Practices in Conducting Medtech Clinical Trials
Clinical trials in the medical technology (medtech) sphere are pivotal in advancing healthcare, requiring meticulous design and execution to ensure their success and validity. To meet the high standards of clinical research, it's imperative to integrate best practices throughout the trial phases, from participant recruitment to data analysis. The medtech industry, through companies like Medtronic, is at the forefront of leveraging technology to enhance trial outcomes.
With a global team of 95,000+ professionals across 150 countries, Medtronic's commitment is evident in their development of medical technologies that impact health every second of the day.
The utilization of technological tools, such as wearable devices and digital patient engagement platforms, has revolutionized how clinical data is collected and analyzed. For instance, the integration of patient monitoring systems and surgical robotics has enabled more precise and real-time data capture, facilitating immediate analysis and identifying safety issues promptly. This innovative approach not only improves patient compliance and reduces data entry errors but also accelerates outcome assessments.
Med4Tech's training program exemplifies the industry's dedication to understanding and addressing the complex needs of clinical medicine. By providing a comprehensive background in medical sciences and exposure to various clinical environments, technology experts are equipped to create more relevant and effective products. This synergy between technology and clinical expertise is vital for fostering future collaborations that will ultimately benefit patients.
In the context of a growing digital landscape where wearable technology users have reached over 1.1 billion, the digitalization of clinical trials is set to rise. The sheer volume of medical data is overwhelming, with a Phase 3 trial now generating an average of 3.6 million data points—three times the amount collected a decade ago. This influx of data, captured and analyzed efficiently, has the potential to drive more successful clinical trials.
However, the balance between innovation and patient safety remains a critical concern, underscored by the rigorous regulatory guidelines governing clinical research. The healthcare industry is navigating these challenges, with regulatory bodies such as the FDA, EU, and EMA proposing new guidelines to manage the risks associated with AI and ML technologies. As the EU AI Act suggests, a risk-based approach is essential for maintaining transparency and upholding ethical standards.
The path of clinical research is complex, requiring a multidisciplinary approach where departments like R&D, Clinical, Quality, Regulatory, and Reimbursement must collaborate effectively. This cooperation is crucial for bridging the gaps between regulatory compliance and market access, as highlighted at the 2024 MedExec Women Conference. The collective understanding of real-world evidence and reimbursement strategies is fundamental for the successful translation of clinical trials into patient benefits.
Ultimately, clinical trials remain a critical component of the research spectrum, providing valuable insights into diseases and enhancing the quality of healthcare. The medtech industry's commitment to harnessing technology and fostering collaboration is pivotal for advancing clinical trials while ensuring the highest standards of patient safety and regulatory compliance.
Challenges and Regulatory Considerations
Conducting medtech clinical trials is a multifaceted endeavor that often encounters significant hurdles. One poignant challenge is the reality of patient recruitment, especially for those with rare diseases who face logistical issues when trials are conducted abroad. For instance, a patient from rural Pennsylvania with an ultra-rare disease may have the chance to join a life-saving trial in Turkey but must navigate the complexities of international travel, visas, and language barriers.
These obstacles underscore the critical need for clinical trial companies to consider the patient experience and provide logistical support.
Technological advancements are also reshaping the landscape of clinical trials. The burgeoning field of artificial intelligence (AI) is revolutionizing data analysis, offering the ability to review unstructured clinical notes with near-human accuracy. This is particularly valuable in conditions like uveal melanoma with liver metastasis, where patients rely on clinical trials due to the absence of FDA-approved treatments.
Despite the potential, AI adoption faces challenges, including integrating the technology into existing workflows and ensuring clinician and public comfort with its use.
Data integrity is another cornerstone of trust in clinical research outcomes. Recent issues, such as the 2022 investigation questioning Alzheimer's disease study results, highlight the importance of meticulous image checking. With manuscripts experiencing a 20-35% rate of image-related problems, it's clear that accidental duplications and errors can slip through, potentially affecting the validity of the research.
Furthermore, the sheer volume of data generated in clinical trials today is staggering. A Phase 3 trial can produce an average of 3.6 million data points, a threefold increase from a decade ago. This influx requires sophisticated data management strategies.
Digital tools like wearable devices and sensors are instrumental in streamlining the trial process by providing real-time data, which enhances outcome assessment and detects safety issues more efficiently.
Yet, the incorporation of technology into clinical trials is not without its difficulties. A multitude of systems and solutions can lead to operational complexity, staff burnout, and elongated research timelines. The challenge is to harness these technological advances while maintaining simplicity and efficiency in the clinical trial workflow.
In conclusion, medtech companies must address these multifaceted challenges—ranging from patient recruitment and logistical support to technological integration and data integrity—to ensure the success and reliability of clinical trials. Each element is essential for advancing medical knowledge and ultimately, improving patient outcomes.
Impact on Patient Outcomes and Healthcare Costs
Clinical trials serve as a pivotal gateway to delivering advanced medical technologies that have the potential to revolutionize patient care. Companies like Medtronic plc are at the forefront, with a bold mission to alleviate pain, restore health, and extend life. They exemplify the transformative impact that clinical trials can have by providing access to life-changing medical technologies.
Medtronic's work across 150 countries, treating 70 health conditions with innovations like cardiac devices, surgical robotics, and patient monitoring systems, underscores the significance of successful trials. Each innovation is a testament to the potential of clinical trials to yield not just medical breakthroughs, but also substantial cost savings.
The healthcare model, once visualized as a four-legged chair comprising patients, providers, plans, and pharmaceutical and medical device companies, is evolving. The introduction of consumer digital apps and the incorporation of digital health strategies by life sciences companies represent this shift. These advancements, fueled by clinical trials, underscore the importance of comprehensive data analysis.
With medical data doubling every 70 days and a single Phase 3 trial generating 3.6 million data points, the depth and breadth of information available to drive clinical decisions and improve patient outcomes are unprecedented.
Furthermore, the narrative of a patient in rural Pennsylvania navigating the complexities of participating in an international clinical trial for an ultra-rare disease illustrates the global reach and profound personal impact of these studies. It's a reminder that behind each data point is a human life, potentially transformed by the technologies and therapies developed through meticulous research and trials. As the clinical trial landscape expands, it's clear that the adoption of innovative, evidence-based medical devices and treatments can lead to better outcomes for patients worldwide and a more efficient healthcare system.
Real-World Examples: Companies Leading the Way
Clinical trial companies are pivotal in driving healthcare innovation, and recent advancements in medical technology have been underpinned by their rigorous research efforts. For instance, the MedTech program is a testament to the burgeoning collaboration between healthcare and technology experts. It equips tech professionals with a robust foundation in clinical medicine, covering everything from anatomy to biochemistry, and immerses them in the clinical environment.
This comprehensive training facilitates the creation of more relevant technological solutions that address real healthcare challenges.
The intersection of technology and healthcare has led to the development of groundbreaking devices, such as AAVAA's brain-computer interface that enables hands-free device interaction for individuals with paralysis. Similarly, Augmental's tongue-controlled 'mouthpad' empowers users with motor impairments to navigate their digital devices effectively. Technologies like these are not just conceptual; they directly contribute to enhancing the quality of life for patients with disabilities.
Proxie is another example where technology serves an essential role in healthcare by providing a platform for families and care providers to efficiently manage home care. Meanwhile, Senbiosys's smart ring represents the integration of CMOS image sensors into wearable tech for non-invasive monitoring of vital health metrics.
Moreover, Kernel's breakthrough in brain health assessment demonstrates how personal health challenges can catalyze innovation. Their user-friendly scanning helmet conceals a sophisticated technology stack for advanced brain measurements, revolutionizing our understanding of mental health and treatment efficacy.
The significant inflow of data is reshaping clinical trials as well. With medical data now doubling every 70 days, Phase 3 trials are generating around 3.6 million data points, highlighting the evolution of data management and analysis in clinical research. This wealth of data not only strengthens the outcomes of clinical trials but also poses a considerable challenge for regulatory professionals to manage.
As technology continues to permeate clinical trials, it introduces novel methods for data collection and patient monitoring, from wearable devices to seamless EMR system integrations. These advancements promise to streamline the trial process, enhance patient engagement, and enable real-time analysis, mitigating the risk of human error. The insights from these technologies are invaluable, providing researchers with the ability to monitor patient outcomes more closely and make informed decisions swiftly.
Yet, the surge in digital health technologies also raises ethical considerations surrounding privacy and the usage of patient data, necessitating a delicate balance between innovation and patient rights. As the role of technology in clinical trials grows, so does the responsibility to navigate these challenges with foresight and integrity.
This synergy of clinical expertise, technological innovation, and ethical vigilance is what propels medtech companies forward, allowing them to make indelible contributions to healthcare and patient well-being.
Future Trends and Innovations in Medtech Clinical Trials
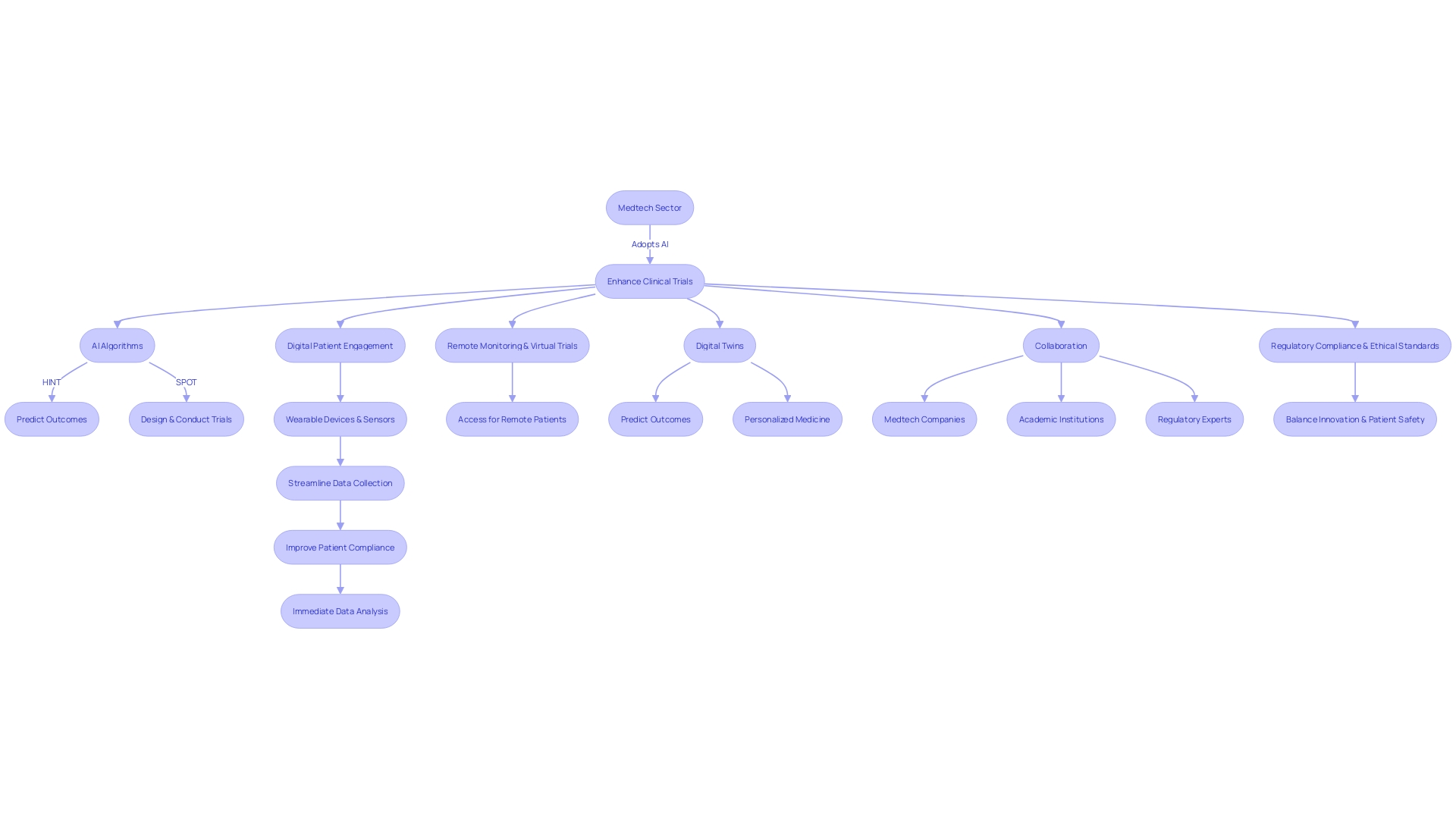
The medtech sector is rapidly adopting cutting-edge technologies to enhance clinical trial methodologies. Artificial intelligence (AI) is revolutionizing the way clinical trials are designed and conducted. For instance, AI algorithms like HINT and SPOT can predict clinical trial outcomes by analyzing drug molecules, target diseases, and patient eligibility criteria.
These tools can shape decisions on trial design or drug development, potentially saving time and resources.
With the amount of medical data doubling every 70 days, clinical trials now generate voluminous datasets, such as an average of 3.6 million data points in Phase 3 trials. This data surge necessitates sophisticated management and analysis tools to ensure trial efficacy and regulatory compliance. The implementation of digital patient engagement tools, wearable devices, and sensors is streamlining data collection, improving patient compliance, and reducing errors, ultimately contributing to more consistent and immediate data analysis.
Moreover, remote monitoring and virtual trials are addressing the challenges faced by patients in accessing clinical studies, especially those in remote locations or with rare conditions. For example, a patient in rural Pennsylvania with an ultra-rare disease now has the possibility to participate in a clinical trial in Turkey, thanks to technological advancements that facilitate cross-border participation.
The future of medtech clinical trials also includes the concept of 'digital twins', mathematical models that replicate real-world processes to predict outcomes. This innovation holds promise for personalized medicine and more efficient trial designs.
As trials become larger and more complex, the collaboration between medtech companies, academic institutions, and regulatory experts is crucial. Programs like the one delivered in partnership with Mecomed and Barts Life Sciences exemplify this trend, providing expert advice and facilitating international connections.
These innovations are not without challenges. The rising complexity of clinical trials requires careful consideration of regulatory compliance and ethical standards. The balance between innovation and patient safety remains a key focus for the FDA and other regulatory bodies as they navigate the integration of AI and other technologies into the medical field.
Additional Resources and References
Clinical trial companies are at the forefront of medtech innovation, navigating a complex landscape of ethical considerations, market incentives, and regulatory hurdles. They must identify and address the intricate ethical, legal, and social implications that emerging technologies bring to the fore. By examining case studies, such as those that present ethical issues through vignettes (such as Box 1 and Box 2), stakeholders can better understand the current challenges and successes within the sector.
Moreover, the real-world problems faced by global pharma companies, like the dilemma encountered by Robin Roberts at Novartis, underscore the critical need for precise evaluation methods in digital health technology applications, where the cost of uncertainty can reach millions.
The UK's commitment to medtech is palpable, as evidenced by programs like the Innovative Devices Access Pathway (IDAP), which aims to streamline the innovation pathway from concept to clinical application. This initiative is part of a broader strategy to provide patients with swift access to medtech solutions, bolstered by the medical technology innovation classification framework that was introduced to standardize the language around innovation. These efforts underscore the importance of medtech throughout the patient care continuum, from prevention to aftercare.
Karen Willcox's work on 'digital twins' and the need for robust mathematical models exemplifies the advances in technology that can potentially transform the future of healthcare. This progress is paralleled by the exponential growth in medical data, which has seen a doubling time decrease from 50 years in 1950 to just 70 days in recent times. The gravity of this data surge is highlighted by the fact that a Phase 3 trial now generates an average of 3.6 million data points, a threefold increase from a decade ago.
Clinical trials, a cornerstone of medical research, are increasingly benefiting from technological enhancements. Digital patient engagement tools, wearable devices, and sensors are revolutionizing trials by expediting outcomes assessment and ensuring data consistency. The integration of technology not only minimizes human error but also accelerates the entire process, paving the way for quicker, more reliable insights into patient health and treatment efficacy.
For those seeking to delve deeper into the realm of medtech clinical trials and their impact on healthcare innovation, a myriad of resources is available. These encompass research papers that dissect the governance of technology across sectors, industry guidelines that provide regulatory direction, and websites offering valuable insights. All these resources collectively support the ever-evolving field of medtech clinical trials, contributing to the enhancement of patient outcomes and the overall healthcare landscape.
Conclusion
Clinical trials play a crucial role in advancing medical innovation by providing essential insights into the safety and effectiveness of new medical technologies. These trials meticulously evaluate devices, procedures, and strategies within real-world clinical settings. The complexity of clinical trials is exemplified by the challenges faced by Robin Roberts at Novartis, who needed to determine the effectiveness of digital health technologies in specific scenarios.
The stakes were high, as incorrect estimations could have significant financial implications.
The evolving landscape of MedTech clinical trials is demonstrated by recent news, with promising projects entering clinical evaluation and companies like Archetype launching to address the alarming statistic that three-quarters of MedTech innovations fail to reach the market. Globalization poses additional challenges for clinical trials, requiring a patient-centric approach that is both scientifically rigorous and accessible. In addition, the volume of data in healthcare is expanding rapidly, necessitating advanced data management and analysis to streamline the trial process.
Technologies such as digital patient engagement tools and wearable devices contribute to more efficient outcome assessments and data consistency.
In conclusion, the advancement of the MedTech sector relies on interdisciplinary collaboration, patient-focused innovation, and strategic market navigation. The complex nature of clinical trials demands a comprehensive understanding of the healthcare ecosystem and adherence to best practices. MedTech companies must address challenges such as patient recruitment, technological integration, data integrity, and regulatory compliance to ensure the success and reliability of clinical trials.
Ultimately, these trials have a profound impact on patient outcomes and the healthcare system as a whole, delivering life-changing medical technologies to those in need.
Experience the power of cutting-edge technologies in clinical trials with bioaccess™




