Introduction
The Importance of Clinical Validation
Clinical validation is a crucial process in the field of medical devices, ensuring their safety and effectiveness. In Latin America, post-market clinical follow-up studies are gaining prominence as these regions strive to uphold high standards for medical devices. Transparency plays a key role in this process, as clear communication of information about the device's intended use, development, and performance is necessary for risk assessment and patient outcomes.
Effective communication strategies are employed to ensure the success of these crucial disclosures. In this article, we will explore the significance of clinical validation, device classification and risk assessment, post-market surveillance, the role of regulatory agencies, case studies of clinical validation in practice, and the challenges and limitations of this process. Join us as we delve into the world of clinical validation for medical devices and gain insights into its importance and implications.
The Importance of Clinical Validation
The process of clinical validation is pivotal for medical devices, particularly regarding their safety and efficacy. This phase is characterized by comprehensive testing and critical analysis of the device's performance, functionality, and reliability. In Latin America, the emphasis on post-market clinical follow-up studies (PMCF) is growing as these regions strive to maintain high standards for medical devices.
Manufacturers are tasked with collecting robust evidence that speaks to the safety profile of their devices, how they should be used, and any potential risks they present. A key aspect of this process is transparency, which involves clear communication of information about the device, including its intended use, development, and performance. This transparency is crucial, as it directly impacts risk assessment and patient outcomes.
It's not just about making information available but ensuring it is accessible and understandable to the intended audience. This means presenting the 'logic' or rationale behind device outputs in an 'explainable' manner, allowing users to comprehend how results are derived or decisions are made. Effective communication strategies are employed, selecting the most suitable media and timing, to ensure the success of these crucial disclosures.
Device Classification and Risk Assessment
In the realm of medical device validation, the classification of a device plays a critical role in shaping the clinical validation process. Devices are placed into different categories based on their associated risks, which can range from low to high.
The risk profile of a device not only influences the intensity of regulatory scrutiny but also dictates the specific clinical data needed for validation. A thorough risk assessment is indispensable as it pinpoints potential hazards and evaluates both the probability of occurrence and the potential severity of harm that could result from the use of the device.
The FDA emphasizes the importance of transparency in this process, which includes clear communication regarding the device's intended use, development, performance, and the logic behind its operation. The concept of explainability is paramount, as it pertains to the capacity to elucidate how a device's output or decision is reached in a manner comprehensible to the intended audience.
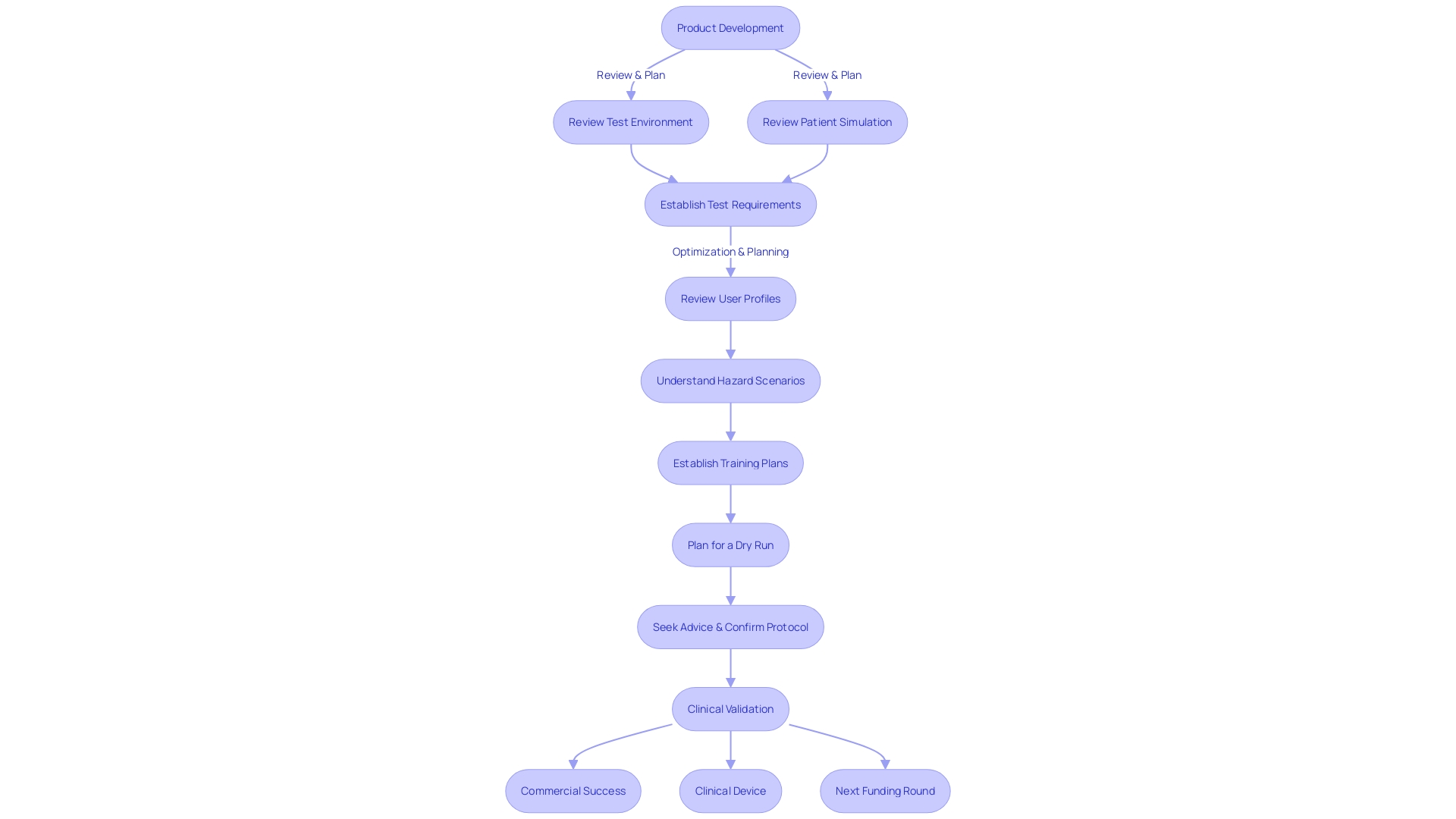
This transparency is crucial as it directly impacts risk assessment and patient outcomes. According to the FDA's guidance on applying human factors and usability engineering to medical devices, simulating the test environment and the patient experience is essential. This simulation should reflect the 'actual conditions and settings in which users interact with the medical device,' as established by IEC 62366-1. The requirements for creating a representative test environment must consider various factors such as lighting, temperature, noise, and potential interference from other devices. By focusing on these elements, researchers can ensure that the device is tested under conditions that closely mimic real-world usage, thereby enhancing the reliability of the validation process.
Post-Market Surveillance and Adverse Event Reporting
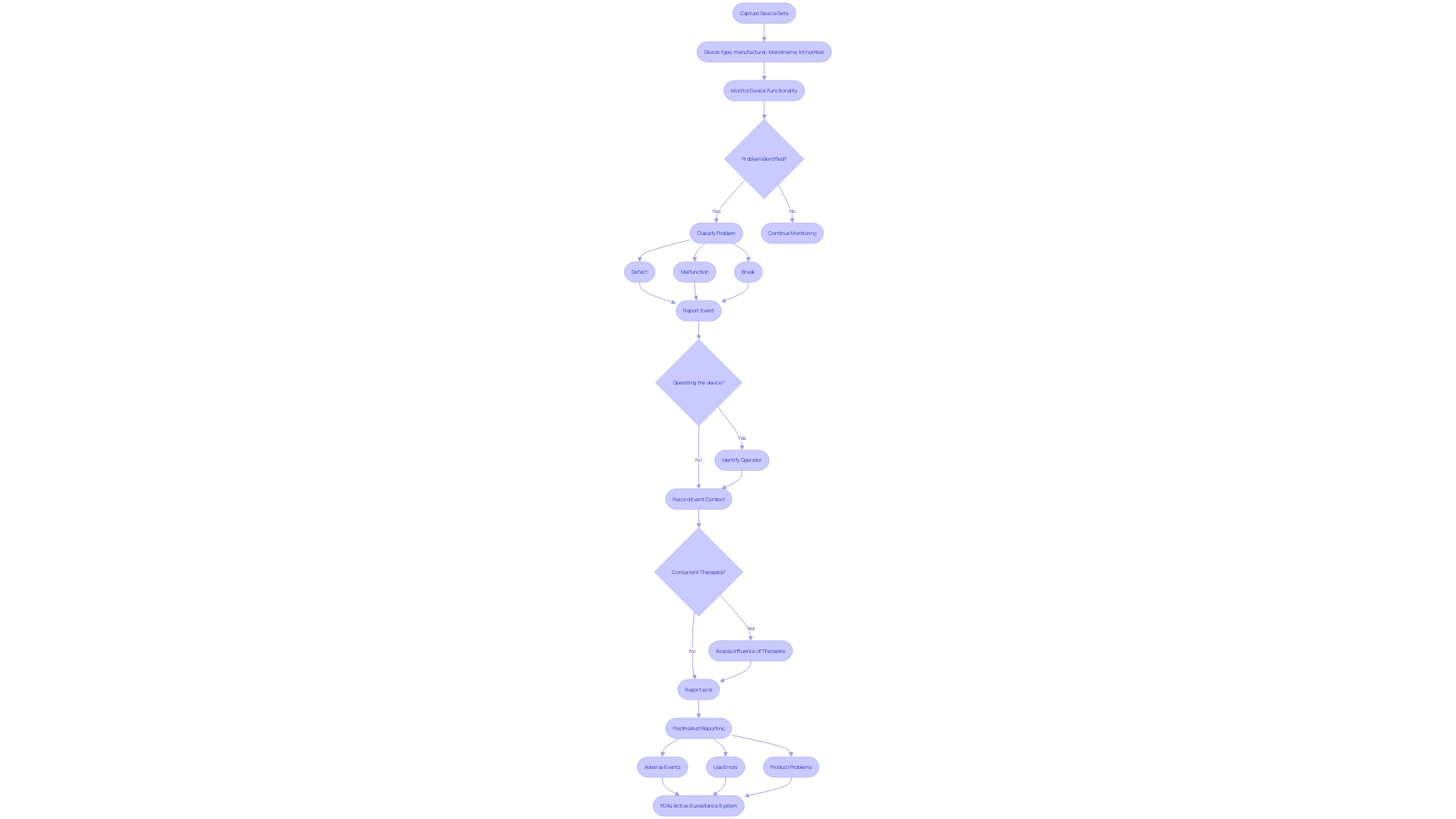
In the realm of medical device safety, post-market clinical follow-up studies serve as a crucial mechanism for ensuring that products continue to perform as intended once they are in use. This ongoing surveillance is essential for capturing data on the device's real-world application, encompassing aspects such as device type, manufacturer, brand name, and lot number. It's not merely about tracking the device's functionality; it's about understanding the context in which any problems may arise.
For instance, discerning whether a device defect, malfunction, or break occurred during operation, and identifying who was operating it, is key. Furthermore, these studies consider whether other concurrent therapies might have influenced any adverse events. The meticulous reporting of such events is fundamental for maintaining the highest standards of patient safety and device efficacy.
As the medical device landscape evolves with increasing regulatory demands, strategies for effective post-market surveillance are being discussed at summits like The MedTech Regulatory Intelligence Summit. Here, professionals delve into best practices for regulatory strategy within the global market. The FDA's active surveillance system initiative, which aims for enhanced data capture and improved detection of safety signals, exemplifies the efforts being made to safeguard public health by ensuring medical devices meet stringent safety and effectiveness criteria.
The Role of Regulatory Agencies in Ensuring Device Safety
In the dynamic field of medical devices, regulatory agencies are the gatekeepers of safety and efficacy. These bodies meticulously scrutinize the clinical data provided by manufacturers, looking beyond the brand name and lot number to assess if any device defects or malfunctions have emerged post-market. The question the context of any adverse events, such as who was operating the device and whether other concurrent therapies may have influenced the event.
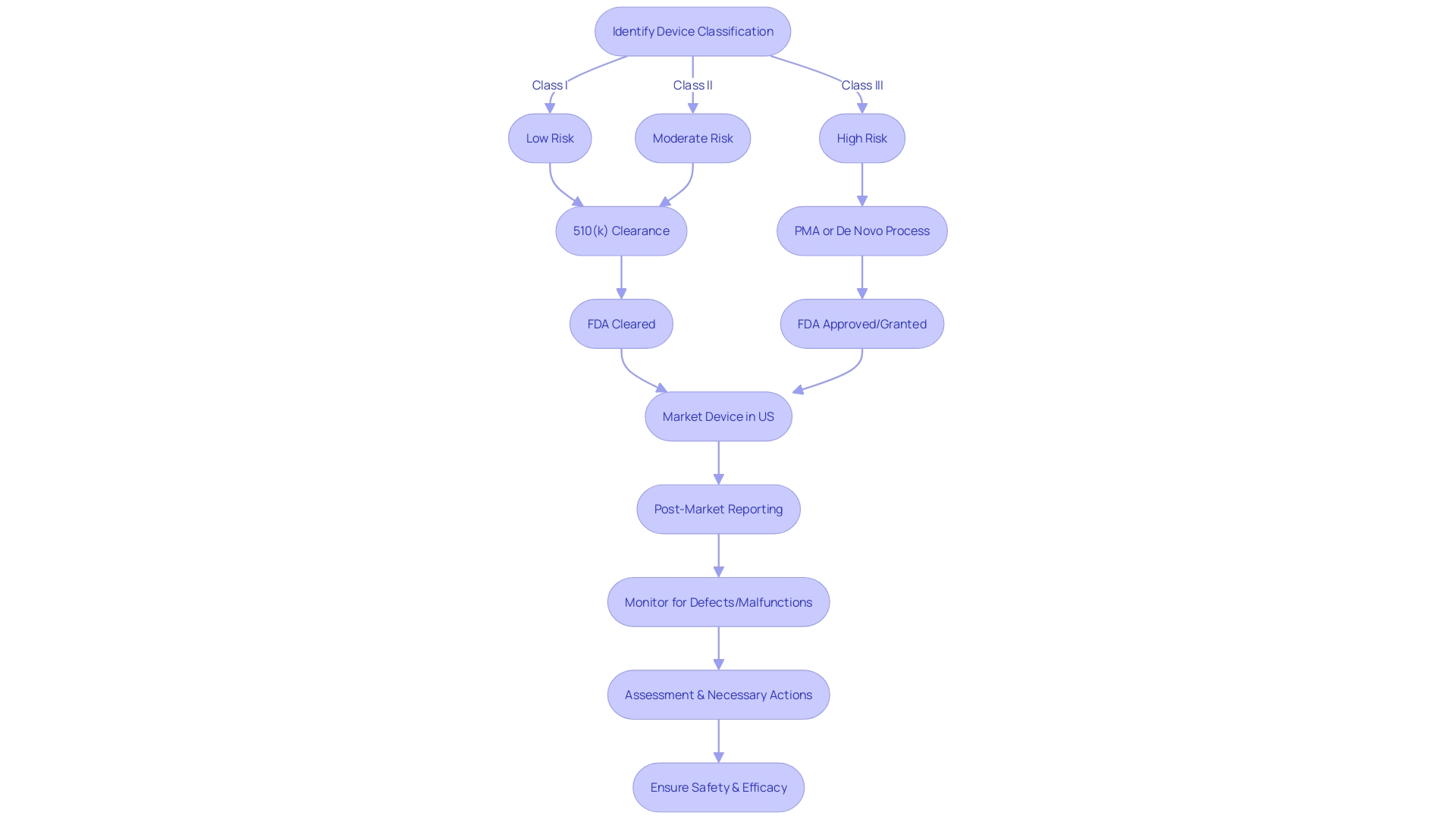
This thorough post-market reporting is essential in capturing use errors and product problems that could have serious implications for patient care. The FDA, a prime example of such an agency in the US, classifies medical devices into three risk-based categories, with class three devices such as life-sustaining pacemakers undergoing the most stringent review processes. Despite representing only about 10% of FDA-regulated devices, their critical nature warrants a longer approval time to ensure the highest level of safety.
Similarly, in Europe, while the EMA plays a role, regulatory control is primarily managed at the Member State level. These agencies must stay abreast of the industry's rapid technological advancements and digitalization, which continually reshape the regulatory landscape. As one expert puts it, the field of radiological health presents unique challenges, such as managing radiation doses and the use of radiopharmaceutical drugs, underscoring the importance of specialized regulatory knowledge in safeguarding medical care.
Case Studies: Examples of Clinical Validation in Practice
The journey of machine learning/artificial intelligence medical devices (Names) to the market is akin to that of new drugs, involving development in the laboratory, followed by rigorous testing for efficacy and safety, and ultimately gaining acceptance from medical professionals and insurers. Despite the allure of technological innovation in Names, their success hinges on rigorous implementation to meet high commercial standards. For instance, the Food and Drug Administration (FDA) in the US categorizes medical devices into three classes, with class three devices such as pacemakers undergoing the most stringent regulatory processes due to their life-sustaining roles.
These high-risk devices represent about 10% of FDA-regulated medical devices and are subject to lengthy approval times. Transparency is critical in this process, with the FDA emphasizing the need for clear communication of information regarding the MAMDs' intended use, development, performance, and logic. Explainability of the device's logic is a key aspect of transparency, ensuring that risks and patient outcomes are appropriately communicated.
This same rigorous approach applies to Digital Health Technologies (DHTs) used in clinical trials for drug development. The FDA's draft guidance on the use of DHTs outlines the importance of selection, verification, validation, and usability of these technologies, acknowledging the balance between consumer-level usability and the need for rigorous data validation. Moreover, the standards and guidance documents such as IEC 62366-1 and the FDA's 'Applying Human Factors and Usability Engineering to Medical Devices' provide a framework for developers to simulate the test environment and patient interactions, thereby optimizing user profiles and understanding critical use scenarios to ensure safety and efficacy.
Challenges and Limitations of Clinical Validation
Clinical validation of medical devices, particularly machine learning/artificial intelligence medical devices (Names), mirrors the stringent process of bringing a new drug to market. It involves development in the lab, followed by comprehensive testing for safety and efficacy.
However, this process can be hampered by the recruitment and retention of trial participants, potentially affecting the representativeness of the results. Addressing the need for a robust testing environment, standards such as IEC 62366-1 provide guidance on simulating real-world conditions, including factors like lighting, temperature, and noise, which must be factored into the testing environment to ensure a credible validation process.
Additionally, the validation of Digital Health Technologies (DHTs) for clinical trials is a critical aspect, as highlighted in a public meeting hosted by the FDA, where the emphasis was placed on the importance of rigorous development and validation of these technologies. Despite the high failure rate of promising drugs, and by extension Names, due to the lofty commercial success criteria, the focus should remain on the successful implementation of these medical devices rather than their technological complexity alone. As such, manufacturers must navigate these challenges with meticulous planning and adherence to validation protocols to uphold the validity and reliability of their clinical validation studies.
Conclusion
To conclude, clinical validation is a crucial process for ensuring the safety and effectiveness of medical devices. In Latin America, post-market clinical follow-up studies (PMCF) are gaining prominence to uphold high standards.
Transparency in communication is vital for risk assessment and patient outcomes. Device classification and risk assessment shape the clinical validation process.
Thorough risk assessment identifies potential hazards and determines the required clinical data based on device categorization. Effective communication strategies ensure clear explanations of device outputs.
Post-market surveillance and adverse event reporting are essential for ongoing device safety. Meticulous reporting captures real-world data and contextual factors, maintaining patient safety and device efficacy.
Regulatory agencies play a critical role in scrutinizing clinical data to assess device defects or malfunctions. Case studies highlight the importance of rigorous implementation and transparency for innovative devices like MAMDs and DHTs. Challenges exist in participant recruitment, simulating real-world conditions, and navigating the commercial landscape. In conclusion, clinical validation involves comprehensive testing, risk assessment, post-market surveillance, and effective communication strategies. Upholding high standards improves patient safety and healthcare outcomes. Collaboration among manufacturers, regulatory agencies, healthcare professionals, and patients is vital for successful clinical validation processes.




