Introduction
Contract Research Organizations (CROs) play a crucial role in advancing medical research by taking on the multifaceted challenges of clinical trial management. These organizations go beyond simply executing research protocols, delving into the ethical, legal, and social intricacies that arise in the dynamic environment of clinical studies.
With a keen eye on market incentives and intellectual property rights, CROs navigate the evolving terrain of emerging medical technologies. They provide the scaffolding for developing new treatments while grappling with the ethical dilemmas presented by each unique case.
In this article, we will explore the role of CROs in advancing medical research, the services they offer, their clients and collaborations, as well as the educational and professional requirements for careers in clinical research. We will also discuss the responsibilities of Clinical Research Associates (CRAs) and the advancement opportunities available in the field. Additionally, we will provide tips for success in clinical research careers and highlight resources and professional organizations that support the growth and development of professionals in this field.
The Role of CROs in Advancing Medical Research
Contract Research Organizations (CROs) are pivotal in the realm of medical advancements, taking on the multifaceted challenges of clinical trial management. These entities are not merely executors of research protocols; they delve into the intricate ethical, legal, and social intricacies that surface in the dynamic environment of clinical studies. With a keen eye on market incentives and intellectual property rights, CROss navigate the evolving terrain of emerging medical technologies.
Through strategic partnerships and careful consideration of a variety of factors, CROss provide the scaffolding for developing new treatments while grappling with the ethical dilemmas presented by each unique case. For instance, consider the plight of a patient from rural Pennsylvania, battling an ultra-rare disease with no approved cure, who is offered a chance to join a clinical trial in Turkey. The patient faces not only the illness but also the daunting task of international travel, language barriers, and bureaucratic hurdles.
This scenario underscores the complex responsibilities CROss bear in facilitating patient access to critical research studies, often across borders. They must consider the international legal and regulatory frameworks, the overarching social objectives of the research aimed at bettering the human condition, and the ethical precedents set by past scientific endeavors, such as the initial HSPC transplants on terminally ill patients. CROs are thus central to bridging the gap between scientific promise and real-world ethical, logistical, and regulatory challenges.
Services Offered by CROs
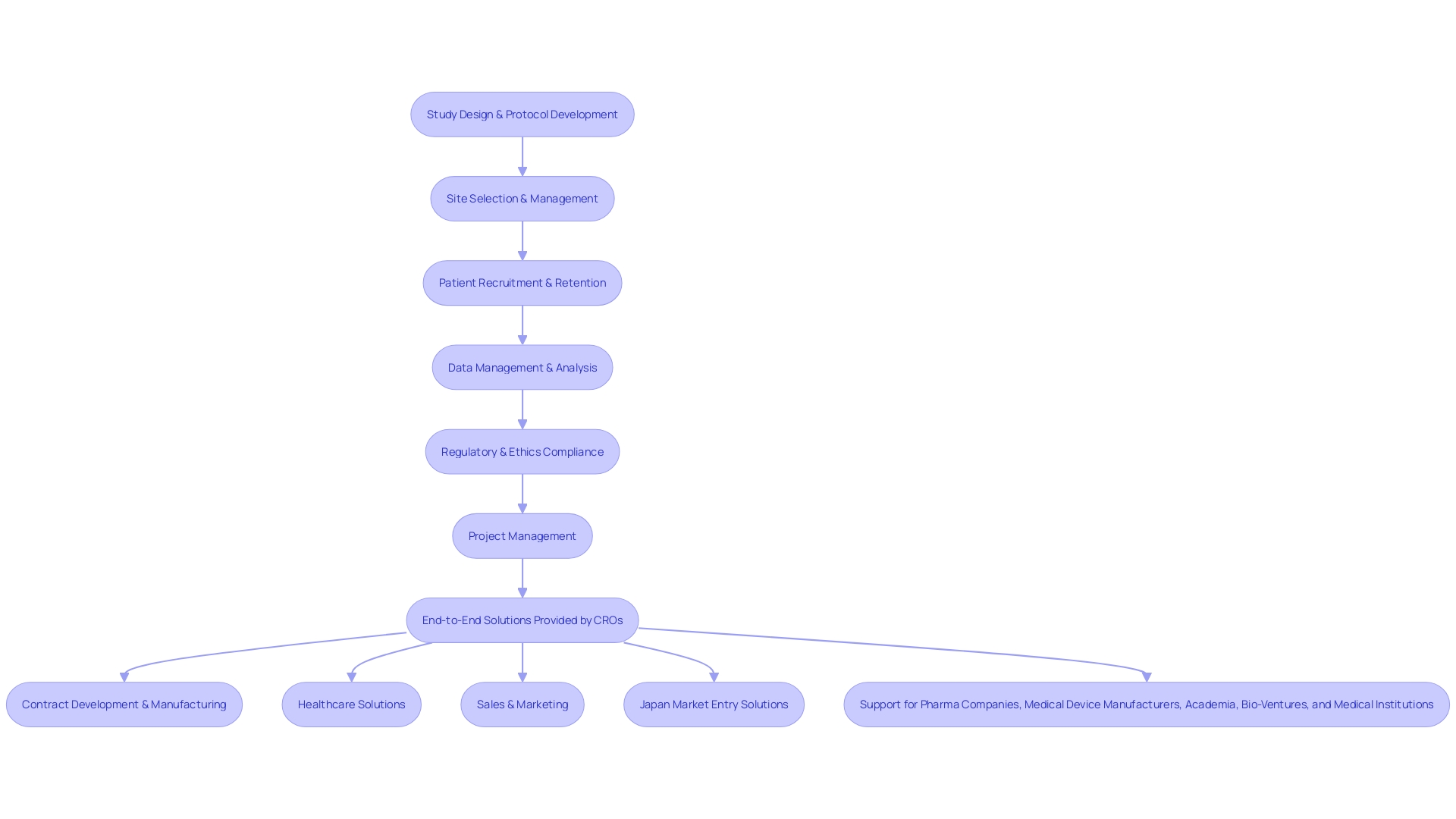
Contract Research Organizations (CROs) are the linchpin in the execution of clinical trials, offering an array of specialized services to ensure the smooth progression from study conception to regulatory approval. These services encompass the entire spectrum of trial management:
1.
Study Design and Protocol Development: CROs like CMIC Group, which initiated the CRO model in Japan, tailor study protocols to align with regulatory mandates and scientific rigor. They ensure that the design is robust, endpoints are meaningful, and the sample size is statistically valid.
- Site Selection and Management: CROs meticulously assess potential study sites, weighing factors such as the patient demographic, facility infrastructure, and adherence to regulatory standards.
They also train and support site staff to guarantee that the study complies with Good Clinical Practice (GCP) guidelines. 3.
Patient Recruitment and Retention: To address challenges like those faced by a patient with an ultra-rare disease in rural Pennsylvania who must navigate international travel to participate in a trial in Turkey, CROss deploy multifaceted strategies for recruiting and retaining participants. They leverage advertising, patient databases, and outreach programs, and also ensure support for logistic concerns and language barriers.
- Data Management and Analysis: CROs harness cutting-edge technology for data management, ensuring the accuracy and integrity of data collection, analysis, and reporting.
This is crucial for creating high-quality reports that will stand up to regulatory scrutiny. 5. Regulatory and Ethics Compliance: Navigating the intricate regulatory landscape, CROs like CMIC ensure that all studies meet the highest standards of ethical conduct and regulatory compliance, assisting with regulatory approvals and the management of adverse events to safeguard participant safety. 6. Project Management: CROs oversee the entire lifecycle of a clinical trial, from planning to execution. They monitor timelines, manage budgets, and facilitate communication among stakeholders, while proactively addressing potential risks. Through these comprehensive services, CROs like CMIC are not just service providers but vital partners in the journey of drug development, ensuring that every 'link in the chain' is optimized to advance medical innovation and patient care.
Clients and Collaborations of CROs
Contract Research Organizations (CROs) have become the linchpin for a multitude of stakeholders in the healthcare and life sciences sectors. They offer comprehensive services that extend beyond traditional clinical trial management, addressing the entire spectrum of the pharmaceutical value chain. This includes contract development, manufacturing, and even market entry strategies.
For instance, CMIC Group in Japan, a trailblazer in the CRO industry, provides customized solutions to meet the specific needs of clients such as pharmaceutical companies, academia, and medical institutions. CROs are adept at navigating complex scenarios, such as facilitating clinical trial participation for patients in remote locations. Consider a patient in rural Pennsylvania with an ultra-rare disease, who has the chance to join a clinical trial in Turkey.
Here, CROss step in to manage intricate logistical challenges, ensuring that the patient's journey to the trial site is as seamless as possible. They assist with travel arrangements, visa procurement, and translation of essential documents, embodying patient-centricity by prioritizing the patient's experience and needs. Embracing diversity, equity, and inclusion (DEI) is central to CROs' ethos, as Daniel J Herron from RWS highlights.
By incorporating a wide array of perspectives and backgrounds into the planning and design of clinical trials, CROs ensure that each patient's voice is heard and their experience is tailored accordingly. This holistic approach is not only about providing support but also about optimizing each decision in the research process, as noted by industry experts who emphasize the importance of meticulous planning years in advance to enhance the efficacy and relevance of clinical trials. These efforts are indicative of the strategic shifts and innovative thinking within the industry, as evidenced by the evolution of companies like Pfizer in their approach to pharmaceutical development.
Careers in Clinical Research
Clinical research careers offer a diverse landscape for professionals eager to contribute to the evolution of healthcare and treatment options. These roles are not limited to one sector but extend across various organizations such as Contract Research Organizations (CROs), pharmaceutical companies, academic institutions, and healthcare facilities. The career paths within clinical research are multifaceted, encompassing roles from clinical research management and project coordination to data analysis, monitoring, regulatory compliance, and medical writing.
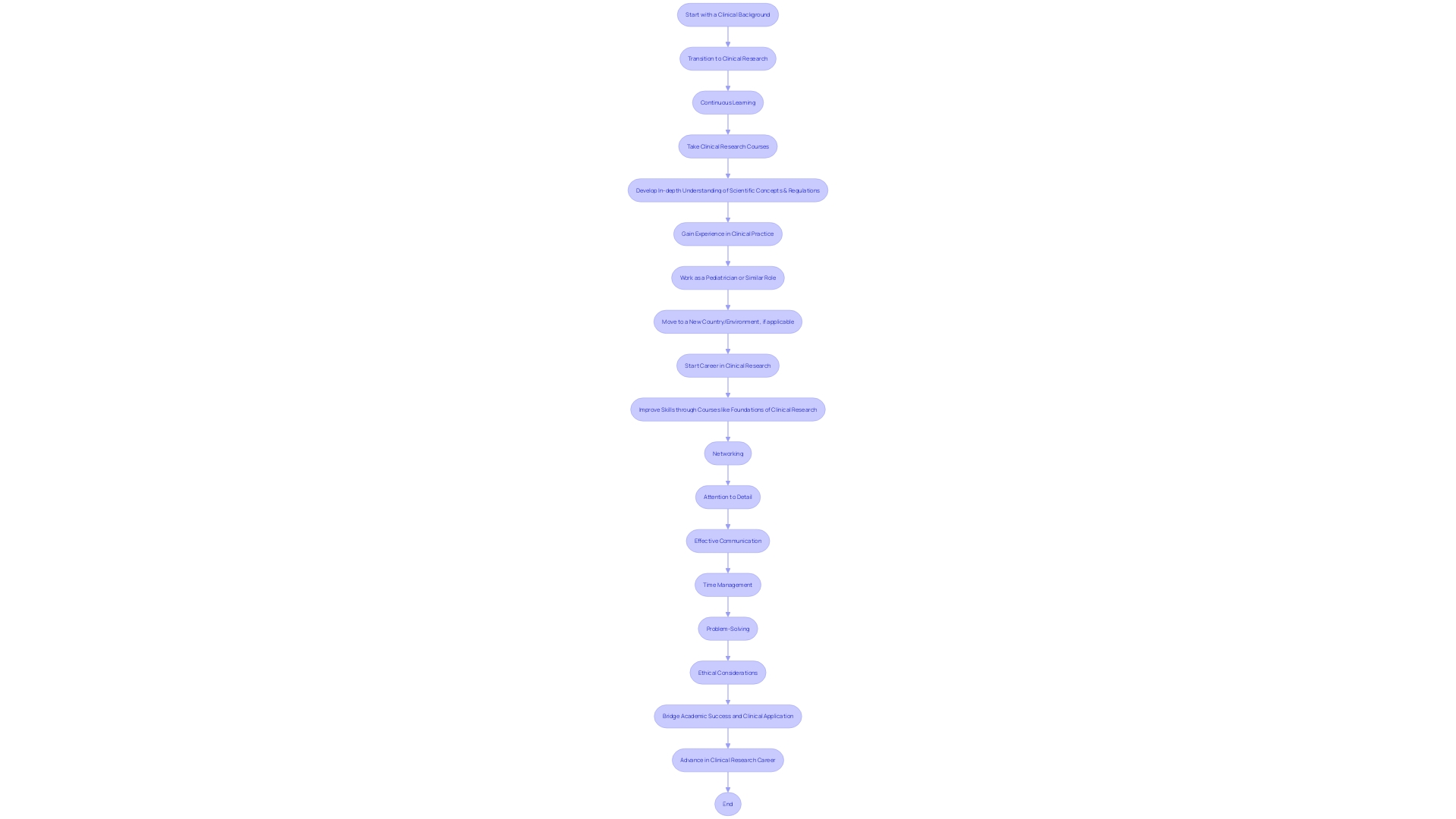
A poignant example of career transition within this field is Dr. Danielyan, who, after a decade as a pediatrician, pivoted to clinical research. Despite her extensive clinical background, she recognized the need to enhance her research acumen and pursued further education at Harvard Medical School’s Foundations of Clinical Research. Similarly, a cancer researcher's role is dynamic and collaborative, requiring a synthesis of existing scientific literature, hypothesis development, and constant communication with a multidisciplinary team to drive forward the fight against cancer.
Moreover, professionals in clinical research are integral to navigating complex scenarios, such as facilitating patient participation in international trials. They must address logistical challenges and ensure the ethical conduct of trials, all while focusing on generating robust data that contributes to the development of innovative therapies. An illustration of such complexity is a patient from rural Pennsylvania navigating the intricacies of joining a trial in Turkey, which underscores the critical role of clinical research professionals in patient support and trial coordination.
Clinical Research Associate (CRA) Roles and Responsibilities
Clinical Research Associates (Cras) play a pivotal role in ensuring the integrity and success of clinical trials, crucial for the advancement of medical knowledge and patient care. Their expertise in scientific concepts and methodologies is vital for the meticulous monitoring of trials and upholding the standards of good clinical practice. Cras are involved in numerous facets of trial management, from the initial selection of study sites, guaranteeing the presence of requisite infrastructure and qualified staff, to the critical phase of study closeout, ensuring all documentation is precise and archived correctly.
During trial monitoring, CRAs undertake rigorous site visits, reviewing documents and verifying trial conduction aligns with protocols and regulatory guidelines. Their responsibilities extend to safeguarding participant safety, overseeing data accuracy, and managing the prompt reporting of adverse events. The insights gained from CRA's work not only contribute to the current trial's success but also help bridge the gap between clinical research and practice, addressing the clinical uncertainties highlighted by the thousands of RCTs registered annually.
In a landscape where Contract Research Organizations (CROss) like CMIC are expanding their services to cover the entire pharmaceutical value chain, Cras are indispensable. They ensure trials are conducted ethically and efficiently, contributing to the end-to-end solutions that CROss provide, from development to market entry. The multifaceted role of Cras is integral to the success of clinical trials and the broader context of medical research and practice.
Educational and Professional Requirements for Clinical Research Careers
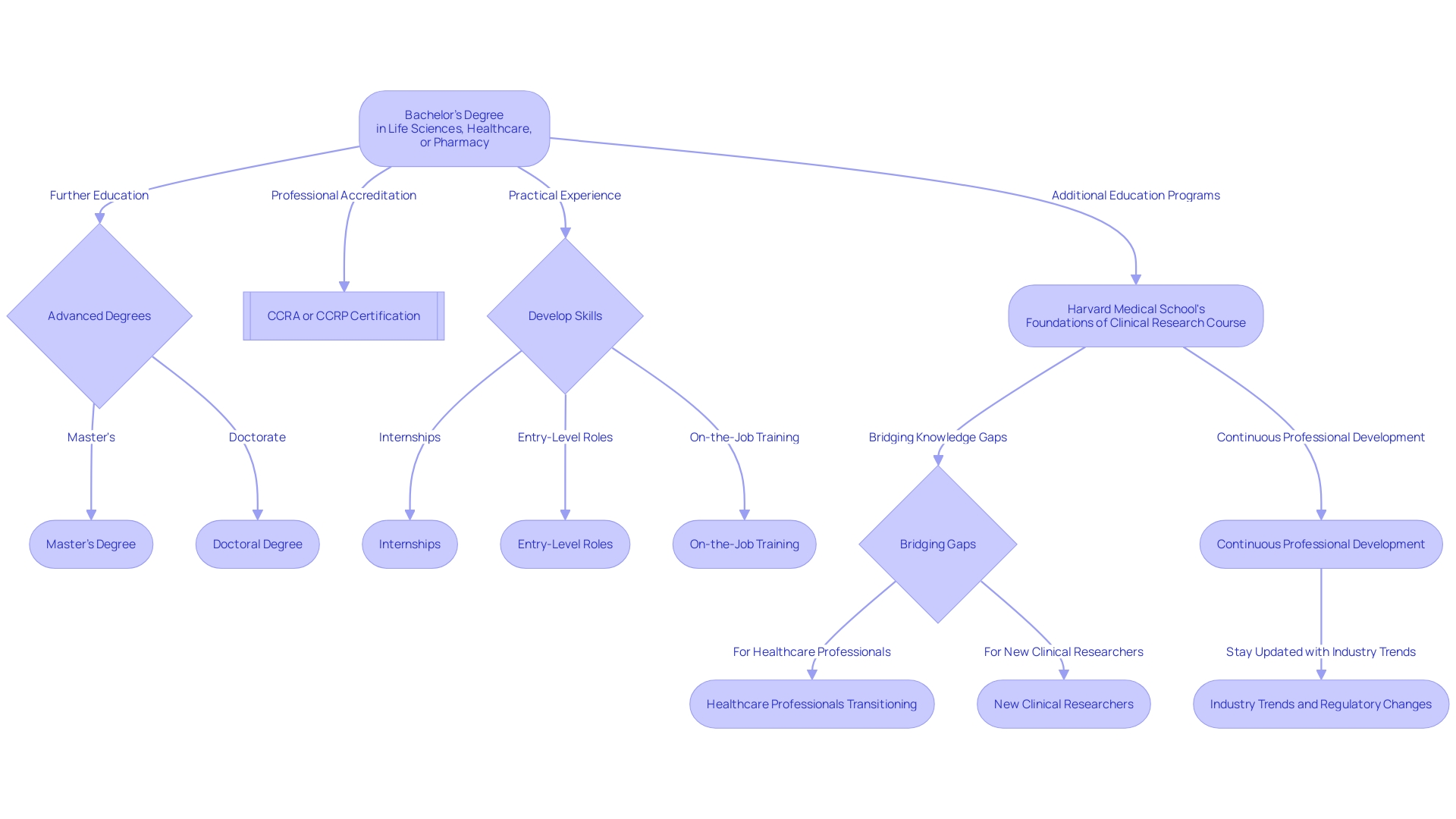
Embarking on a career path in clinical research demands a robust foundation of education paired with hands-on experience. Typically, entry into this field begins with a bachelor's degree in a pertinent discipline, such as life sciences, healthcare, or pharmacy.
Some roles may necessitate further academic qualifications, including master's or doctoral degrees. Professional accreditations, for instance, the Certified Clinical Research Associate (CCRA) or Certified Clinical Research Professional (CCRP), are valuable in signaling expertise and dedication to the profession.
Practical skills are honed through internships, entry-level roles, and on-the-job training. For those transitioning from different areas of healthcare, like Dr. Danielyan, who shifted from pediatrics to clinical research, additional education such as Harvard Medical School's Foundations of Clinical Research course can bridge knowledge gaps.
This course, which addresses safety concerns and specific population needs in drug development, is crucial for healthcare professionals and those in biomedical research. It equips participants with a practical understanding of medical product safety and the intricacies of clinical trials. Mastery in integrating scientific knowledge, formulating research questions, and effectively communicating findings are indispensable skills. Continuous professional development and staying abreast of industry trends and regulatory changes are pivotal for a successful career in clinical research.
Advancement Opportunities in Clinical Research
The field of clinical research not only promises significant contributions to medical advancements but also presents a landscape rich with professional growth. Individuals who immerse themselves in this realm have the potential to ascend to pivotal positions by deepening their expertise and embracing leadership responsibilities.
For example, Dr. Danielyan's journey from a seasoned pediatrician to an esteemed associate clinical scientist at Agenus showcases the transformative power of specialized education, such as the Foundations of Clinical Research course offered by Harvard Medical School, in bridging the gap between clinical experience and research proficiency. This commitment to learning and adaptation is emblematic of the career trajectory in clinical research, where roles like Clinical Research Manager, Project Manager, Clinical Operations Director, and Regulatory Affairs Manager become attainable through demonstrated leadership, a robust understanding of scientific methodologies, and adherence to the stringent regulations that govern clinical practice. Such advancements not only elevate individual careers but also fuel the collective endeavor to forge new paths in therapeutic innovation.
Tips for Success in Clinical Research Careers
Clinical research is a field that demands a fusion of scientific knowledge, regulatory awareness, and practical skills. For instance, consider the journey of Dr. Danielyan, who, after a decade as a pediatrician, transitioned into clinical research. Recognizing her lack of experience in this new domain, she wisely pursued Harvard Medical School’s Foundations of Clinical Research course, bridging the gap between her extensive clinical background and the research knowledge required for her role at Agenus.
Professionals in clinical research must maintain a rigorous commitment to continuous learning to stay abreast of industry trends and regulations, which are critical for patient safety and product development. Workshops, certifications, and specialized training courses are indispensable for healthcare professionals transitioning to this field. Networking is not just about building contacts; it’s about engaging with a community that includes regulators, industry peers, and healthcare professionals.
The collective wisdom of this network is invaluable in navigating the complex landscape of clinical trials. In clinical research, precision is paramount. Whether it's reviewing protocols or scrutinizing data, attention to detail can make or break a study's integrity.
Furthermore, effective communication is the cornerstone of collaboration with stakeholders, as highlighted in a recent Flatiron publication, which emphasized the importance of data quality and reliability for scientific inferences. Time management and problem-solving are equally essential, as clinical trials often juggle multiple projects with pressing deadlines. Ethical considerations are the bedrock of research integrity, ensuring the safety of participants and the credibility of data.
As underscored by the challenges of translating research into clinical practice, such as the underutilization of cancer subtypes in patient care, it is clear that there is a gap between academic success and clinical application. This was further emphasized in a JAMA special communication, which noted the disconnect between clinical trials and clinical practice, underscoring the need for clinical researchers to develop skills that bridge this divide. In essence, a successful clinical researcher combines a deep understanding of scientific principles with a practical approach to addressing the unique challenges of the field, always guided by ethical standards and a commitment to advancing medical knowledge.
Resources and Professional Organizations
Professionals in the clinical research arena have access to a wealth of organizations and databases that support the enhancement of their expertise and career development. The Association of Clinical Research Professionals (ACRP) is a cornerstone in this landscape, offering a suite of educational, training, and certification opportunities designed to foster excellence in clinical research.
Similarly, the Society for Clinical Research Sites (SCRS) serves as a hub for clinical research sites, facilitating productive partnerships between these sites and industry entities. In the realm of regulatory compliance and global guidelines, the International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) provides essential directives for the ethical and methodological conduct of clinical trials.
Complementing these guidelines is the expansive repository of ClinicalTrials.gov, which houses a detailed compilation of clinical trial data, from study designs and participant requirements to outcomes and findings. Moreover, regulatory agencies such as the FDA and EMA are pivotal, supplying comprehensive regulations and guidance for the execution of clinical trials.
These resources, when leveraged effectively, enable clinical research professionals to remain abreast of industry standards, enhance their skill sets, and contribute meaningfully to the advancement of medical research. The importance of these resources is underscored by the challenges faced in translating research into clinical practice, as highlighted by the difficulties in implementing new classification schemes for diseases like colon cancer. Additionally, the separation between clinical trialists and clinicians often leads to inefficiencies, as noted by Gregory Curfman and Derek Angus in their discussions on integrating clinical trials with clinical practice. By staying engaged with these professional resources, individuals in clinical research can help bridge the gap between trial results and real-world medical applications.
Conclusion
In conclusion, Contract Research Organizations (CROs) play a crucial role in advancing medical research by navigating the complex challenges of clinical trial management. They provide comprehensive services such as study design, site selection, patient recruitment, data management, regulatory compliance, and project management.
CROs collaborate with stakeholders in the healthcare and life sciences sectors to develop customized solutions for pharmaceutical companies, academia, and medical institutions. They prioritize patient-centricity and incorporate diverse perspectives into trial planning.
Careers in clinical research offer diverse opportunities across organizations like CROs, pharmaceutical companies, academic institutions, and healthcare facilities. Continuous education through academic qualifications and professional accreditations is crucial for success.
Clinical Research Associates (CRAs) are essential for ensuring the integrity of clinical trials. Their expertise enables meticulous monitoring of trials and upholding good clinical practice standards.
To excel in clinical research careers, professionals must commit to continuous learning and possess effective communication skills, time management abilities, problem-solving capabilities, and ethical considerations. Resources such as professional organizations like ACRP and SCRS provide support through educational programs and certifications. Regulatory agencies like FDA and EMA offer guidelines for conducting trials. In summary, CROs are instrumental in advancing medical research by managing complex challenges while prioritizing patient-centricity. Careers in clinical research offer diverse opportunities for professionals dedicated to continuous learning. By embracing leadership responsibilities and utilizing available resources effectively, individuals can contribute meaningfully to medical advancements while ensuring adherence to ethical standards throughout their careers.




