Overview
This article underscores the pivotal role of clinical trials in revolutionizing the Medtech industry by validating the safety and efficacy of innovative medical technologies. It asserts that these trials not only facilitate regulatory approval but also significantly enhance patient care through improved outcomes. Transformative studies in areas such as:
- drug-eluting stents
- insulin pumps
- minimally invasive surgical techniques
exemplify the critical function of clinical research in advancing healthcare solutions. By illustrating these advancements, the article establishes a compelling narrative about the importance of clinical trials in shaping the future of medical technology.
Introduction
In the rapidly advancing world of medical technology, clinical trials stand as the cornerstone of innovation, ensuring that new devices are not only effective but also safe for patient use. These meticulously structured studies pave the way for groundbreaking advancements, transforming concepts into real-world applications that can significantly enhance patient care.
As the Medtech sector encounters unique challenges, grasping the role of clinical trials becomes essential in navigating regulatory landscapes and fostering trust among stakeholders. This article explores the critical importance of clinical trials in Medtech, examining their impact on innovation, the various types of trials that shape medical solutions, and the transformative outcomes they yield for patient health and the broader healthcare ecosystem.
Understanding the Role of Clinical Trials in Medtech Innovation
Medtech clinical trial examples are pivotal in validating the safety and efficacy of medical technologies, serving as a structured framework for testing new devices. These assessments guarantee that innovations not only satisfy regulatory criteria but also offer concrete advantages to healthcare. In the Medtech sector, these trials facilitate the vital shift from concept to market, allowing for a comprehensive assessment of new technologies in real-world settings.
This rigorous assessment process enhances product development and builds trust among stakeholders, including healthcare providers and patients, by showcasing the reliability of new medical devices through evidence-based results, such as Medtech clinical trial examples.
The importance of medical research in Medtech innovation is underscored by recent statistics showing that 54% of biopharma participants emphasize simplifying enrollment in patient-support programs, a priority that is significantly less among Medtech participants at merely 26%. This disparity highlights the necessity for customized approaches in research design that address the distinct challenges faced by Medtech companies. Effective risk assessment requires cross-functional team planning and alignment on terminology among stakeholders, which is essential for overcoming these challenges.
Furthermore, applying risk-oriented methods in research has been demonstrated to enhance data quality, boost resource efficiency, and reduce project timelines, ultimately accelerating the time to market for new devices. For instance, bioaccess® specializes in managing various types of studies, including Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Follow-Up Studies (PMCF). With over 20 years of experience in the Medtech sector, bioaccess® offers specialized expertise and adaptability to the research process, enabling a streamlined method that can significantly decrease the time and resources required for successful completion.
Expert opinions further reinforce the significance of Medtech clinical trial examples in the realm of innovation. As noted by Vivienne van der Walle, Founder and Medical Director, 'Anything that takes away time from individuals is a pain point for a site, and anyone who resolves that is aiding care.' This perspective emphasizes the necessity for ongoing advancement in study design to improve participant experience and results.
In summary, research studies, including Medtech clinical trial examples, are not merely regulatory obstacles; they are vital elements of the Medtech innovation ecosystem, propelling advancements that ultimately enhance patient care and health outcomes. With bioaccess® at the forefront of clinical research in Latin America, the emphasis on innovation and regulatory excellence is poised to amplify the effects of these studies on local economies, promoting job creation and healthcare advancement.
Case Studies: Pioneering Clinical Trials in Medtech
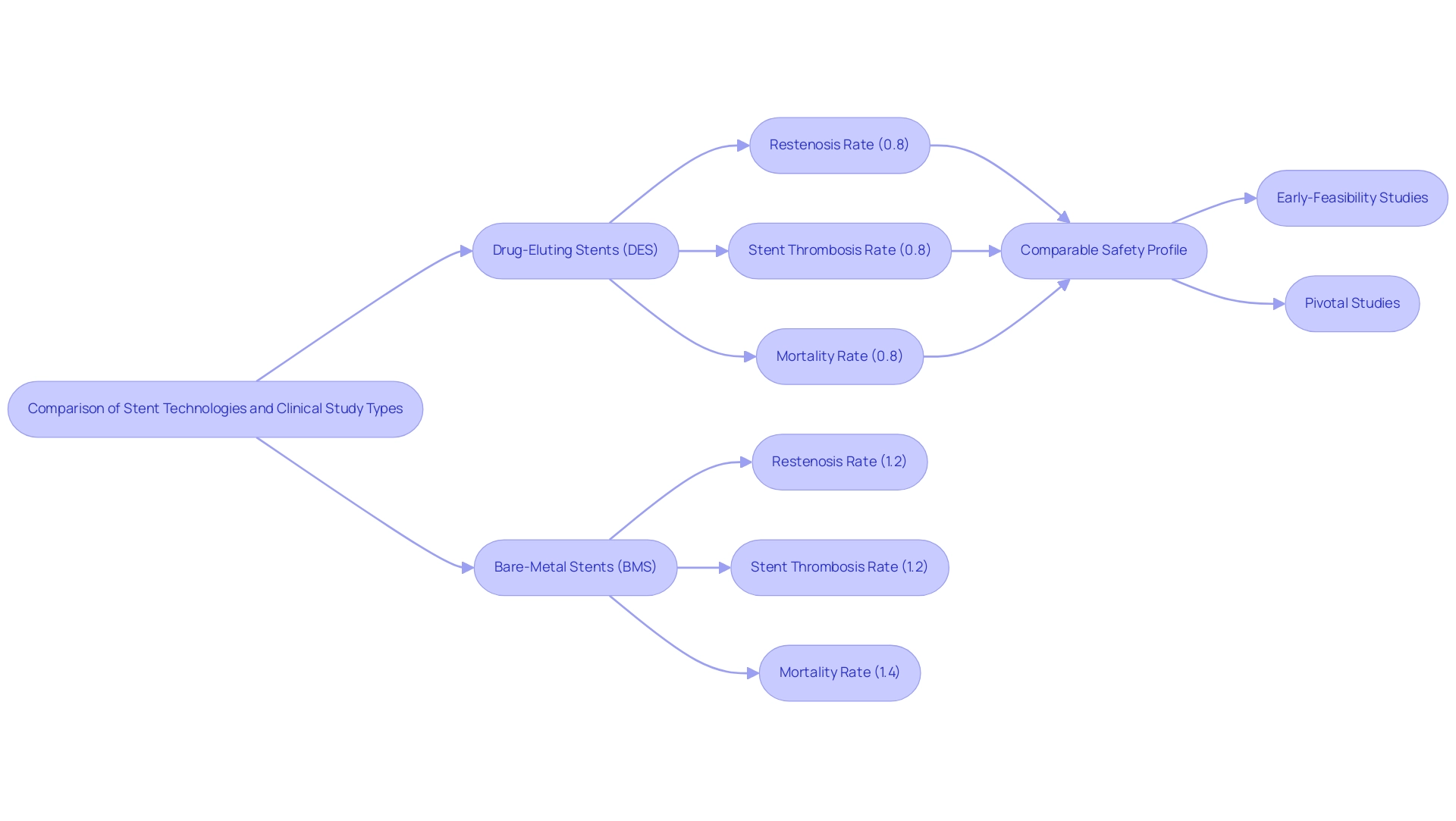
The advent of the first drug-eluting stent (DES) represents a pivotal advancement in cardiac care. Clinical studies have demonstrated that DES significantly reduces restenosis rates compared to traditional bare-metal stents, with stent thrombosis rates reported at 0.8% for DES versus 1.2% for conventional treatments. Furthermore, mortality rates associated with DES stand at 0.8%, compared to 1.4% for conventional treatment, indicating a comparable safety profile between these strategies. This compelling evidence has catalyzed the widespread adoption of DES, leading to enhanced patient outcomes and a paradigm shift in the management of coronary artery disease.
As noted by Masahiro Natsuaki, "Advantages of Nobori BP-BES over DP-EES were not apparent even at five-year follow-up after stent implantation," underscoring the continuous evaluation of stent technologies.
In this context, bioaccess® offers comprehensive management services for clinical studies, including Medtech trial examples such as:
- Early-Feasibility Studies (EFS)
- Pivotal Studies
ensuring that these innovations undergo rigorous testing and validation in the Latin American market. With over 20 years of experience in Medtech, bioaccess® combines specialized expertise and adaptability to effectively navigate the complexities often encountered in Medtech clinical trial examples.
Types of Clinical Trials That Shaped Medtech Solutions
Key studies are pivotal in the regulatory approval process for medical devices, as they are specifically designed to provide definitive evidence regarding a device's safety and efficacy. These studies often involve larger patient populations and are conducted under stringent regulatory guidelines. In 2025, the significance of these studies will be underscored by the FDA's anticipated introduction of the Single IRB Requirement, aimed at streamlining the review process across various locations.
The formalization of this requirement will necessitate adjustments to standard operating procedures and resource allocation for some institutions, thereby enhancing the efficiency of key studies and ultimately accelerating the path to market for innovative devices. At bioaccess®, we leverage our 20+ years of Medtech expertise to ensure that these studies are conducted with the utmost precision and compliance, facilitating a smoother regulatory journey.
Initial feasibility evaluations are conducted during the early phases of device development to assess the practicality and potential clinical utility of new technologies. These investigations typically involve a small patient group and are crucial in identifying any potential challenges before larger-scale trials. Recent statistics indicate that early-feasibility assessments are gaining traction in the Medtech sector, with over 30% of Medtech companies now integrating these evaluations into their development strategies.
This trend reflects a proactive approach to innovation, as companies strive to mitigate risks early in the development process. Bioaccess® specializes in managing these early-feasibility assessments, providing tailored solutions that align with the unique needs of each client.
First-In-Human Studies and Pilot Studies are essential components of the clinical trial landscape, providing Medtech clinical trial examples for the initial evaluation of new devices in human subjects and assessing their performance in a controlled environment. At bioaccess®, we ensure that these analyses are meticulously planned and executed, drawing on our extensive experience to support our clients' innovative endeavors.
PMCF evaluations are critical for monitoring the long-term safety and effectiveness of medical devices post-regulatory approval. These analyses ensure ongoing compliance with regulatory standards and yield valuable data on the real-world performance of devices. As the Medtech landscape evolves, the importance of PMCF studies is increasingly recognized, with regulatory bodies emphasizing the need for continuous monitoring to ensure safety.
By implementing robust PMCF strategies, companies can not only meet regulatory requirements but also enhance their reputation and trust among healthcare providers and patients alike. As Max Baumann, Head of Execution at Treehill Partners, remarked, "Entering 2025, we persist in observing biotech encountering essential business model difficulties as medical end-markets grow increasingly congested," highlighting the necessity for efficient research design in this competitive landscape. Furthermore, the trend of sponsors insourcing data management processes is transforming the industry, enabling enhanced control and transparency over research data, which is vital for operational efficiency and data quality.
At bioaccess®, we are committed to providing comprehensive research management services in Latin America, ensuring that every aspect of the process is meticulously handled to support our clients' success.

Example 1: Breakthrough Innovations from Pivotal Trials
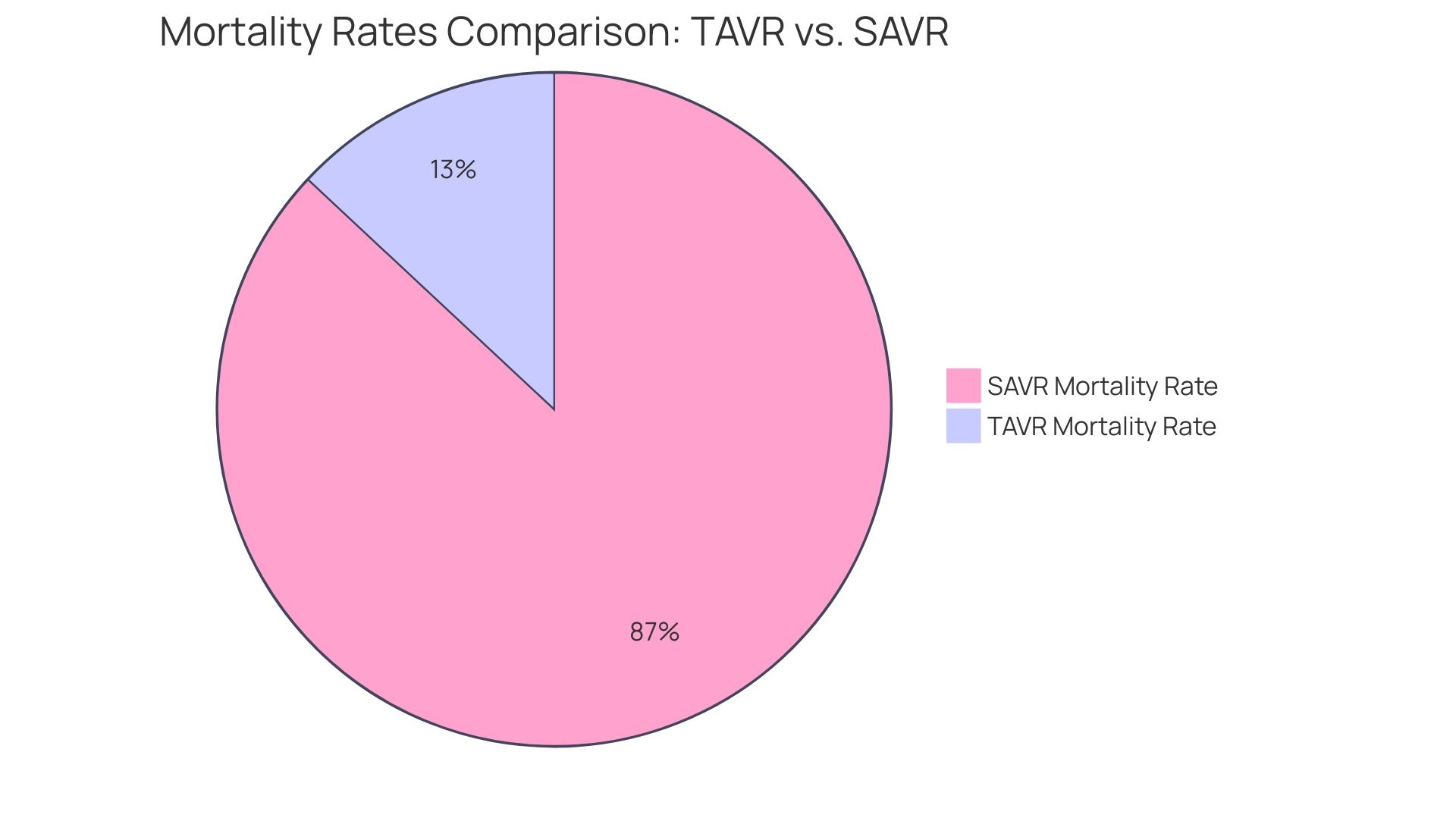
A landmark example in Medtech clinical trial research is the pivotal study for the first transcatheter aortic valve replacement (TAVR) device. This groundbreaking study revealed that TAVR significantly lowered mortality rates in individuals suffering from severe aortic stenosis compared to traditional surgical approaches. Specifically, the study demonstrated a mortality rate of just 1.5% for TAVR, contrasting sharply with the 10.0% rate observed in surgical aortic valve replacement (SAVR) procedures. Moreover, the rate of significant structural valve deterioration (SVD) was also remarkably different, with 1.5% for TAVI and 10.0% for SAVR.
The success of the TAVR study not only enabled the device's rapid approval but also transformed the treatment approach for individuals at high risk. By facilitating less invasive procedures, TAVR has contributed to improved recovery times and overall individual outcomes. Dr. Yeh observed that the extrapolated real-world treatment effects of TAVR have demonstrated benefits akin to those seen in research studies, reinforcing its role as a transformative option in the management of aortic stenosis.
Furthermore, a comprehensive study published in The Annals of Thoracic Surgery analyzed data from over 42,000 patients who underwent SAVR, establishing a five-year survival rate of 93%, with nearly 90% surviving to eight years. This benchmark underscores the effectiveness of surgical interventions while highlighting the evolving landscape of aortic valve treatments, where TAVR stands out as a pivotal advancement. To achieve similar success in clinical studies, utilizing comprehensive clinical study management services like those provided by bioaccess® is essential.
With over 20 years of experience in Medtech and expertise in Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF), bioaccess® offers the specialized knowledge and flexibility necessary to navigate the complexities of medical device evaluations in Latin America. Their customized strategy guarantees adherence, efficient study setup, and effective project oversight, ultimately contributing to enhanced healthcare results and advancing the Medtech sector. For instance, bioaccess® has effectively conducted studies that led to notable progress in device authorization and healthcare, showcasing their ability to enable results similar to those observed in the TAVR study.
Example 2: Transformative Clinical Trials in Device Development
Clinical studies for the first fully implantable artificial heart represent a significant milestone in transformative innovation within the Medtech sector. These experiments have encountered substantial challenges, particularly concerning device performance and user safety. For instance, the longest duration an individual has lived with an artificial heart is 21 months, highlighting the urgent need for advancements in device reliability and outcomes.
As Alain F. Carpentier stated, "A completely implantable artificial heart will be prepared for medical testing by 2011 and for alternative transplant in 2013," underscoring the historical expectations surrounding these innovations.
The data gathered during these experiments facilitated critical design enhancements, resulting in a device capable of supporting individuals for extended periods while they awaited heart transplants. This innovation not only advanced cardiac care but also established new benchmarks for future device development. Beginning in 2025, ongoing advancements in cardiac care continue to emerge from these research studies, demonstrating a commitment to enhancing safety and effectiveness in high-risk medical devices.
Moreover, the integration of advanced methodologies, such as federated learning, has shown promise in refining predictive models for cardiovascular disease management while addressing privacy concerns. This approach exemplifies how collaboration among healthcare organizations can yield improved health outcomes. The insights gleaned from these Medtech clinical trial examples are invaluable, paving the way for future innovations that prioritize patient safety and effective treatment options in the domain of artificial hearts.
In this landscape, bioaccess® emerges as a leading contract research organization facilitating medical device studies in Latin America. With over 20 years of experience, bioaccess® offers comprehensive clinical research management services, including:
- Feasibility assessments
- Site selection
- Compliance reviews
- Setup
- Import permits
- Project management
- Reporting
Their focus on Medtech clinical trial examples, encompassing early feasibility, first-in-human, pilot, pivotal, and post-market follow-up research, ensures that innovations like the artificial heart can advance more swiftly and effectively.
Furthermore, understanding the regulatory landscape, particularly the role of INVIMA as a Level 4 health authority, is crucial for navigating the complexities of medical device evaluations in Colombia. Additionally, the impact of medical studies on local economies, including job creation and healthcare improvement, underscores the importance of bioaccess®'s contributions to fostering international collaboration and economic growth. Bioaccess® is dedicated to accelerating the advancement of medical devices through its expertise and tailored approach, reinforcing the relevance of these discussions to the target audience.
Example 3: Clinical Trials That Redefined Patient Care
The clinical studies for the first generation of insulin pumps represent a pivotal shift in diabetes management, showcasing the profound impact of continuous insulin delivery on blood glucose regulation. These studies not only confirmed the efficiency of insulin pumps but also spurred their broad acceptance, fundamentally transforming care for individuals. Consequently, healthcare providers began to adopt more personalized diabetes management strategies, tailoring treatment plans to individual needs.
This shift has led to significant improvements in the quality of life for individuals living with diabetes.
In 2025, the effectiveness of insulin pump studies is underscored by compelling statistics: individuals using automated insulin delivery systems experienced a reduction in the percentage of time with glucose readings exceeding 180 mg/dL, with only 39.1% of readings in the automated group compared to 44.8% in the control group. Such data highlights the advancements in diabetes management technologies and their role in enhancing patient outcomes. Data management and analysis were conducted using R software, with continuous glucose monitoring data extracted from Nightscout, further adding credibility to these findings.
Moreover, the collaboration between Medtronic and Abbott to develop an integrated continuous glucose monitoring (CGM) system exemplifies the ongoing innovation in this space. This partnership aims to address user concerns regarding sensor design, ultimately improving acceptance and adoption of Medtronic's insulin pumps in diabetes management. The anticipated outcome of this collaboration is to enhance user acceptance of Medtronic pumps, potentially increasing their adoption in diabetes management.
In this context, leveraging the expertise of specialized clinical study management services, such as those provided by bioaccess®, can significantly enhance the success of Medtech clinical trial examples. With over 20 years of experience in Medtech, bioaccess® focuses on managing various Medtech clinical trial examples, including:
- Early-Feasibility Studies (EFS)
- First-In-Human Studies (FIH)
- Pilot Studies
- Pivotal Studies
- Post-Market Clinical Follow-Up Studies (PMCF) in Latin America
Their comprehensive approach ensures that trials are conducted efficiently, adhering to compliance standards while fostering international collaboration that can lead to job creation and economic growth in local communities.
Expert opinions further reinforce the significance of these advancements. As noted by Que Dallara, EVP and President of Medtronic Diabetes, "We're incredibly proud that the MiniMed 780G system continues to be the most widely used automated insulin delivery system in Europe since we launched it in 2020." This evolution of insulin pump technology continues to influence personalized diabetes management strategies, ensuring that individuals receive tailored care that meets their unique needs.
The ongoing research and development in this field promise to revolutionize diabetes management, making it more effective and accessible for individuals globally.

Example 4: Innovative Approaches in Clinical Research
Adaptive trial designs have emerged as a transformative approach in the development of new cancer therapies, showcasing innovative methodologies in clinical research. By allowing alterations to the research protocol based on interim findings, these designs enable investigators to implement real-time changes that significantly improve participant outcomes. A notable case analysis involving a two-stage Phase III design with population enrichment effectively demonstrated how this adaptive approach reduced false negatives for heterogeneous treatment effects.
Facilitating subgroup selection according to established criteria, the study enhanced the precision of results across various demographics. The inherent adaptability of flexible studies has not only accelerated the approval process for effective treatments but has also notably influenced care for individuals. In 2025, statistics reveal that adaptive research designs have led to a remarkable boost in the speed of introducing new cancer therapies to market, with some analyses reporting a decrease in time to approval by as much as 30%. This expedited process is especially vital for individuals facing limited treatment alternatives, as it enables faster access to potentially life-saving therapies.
Furthermore, expert insights underscore the advantages of adaptive studies in medical research. Joan Busner, Clinical Vice President, noted that recent efforts, such as developing an abbreviated PANSS for adolescent use, aim to improve treatment sensitivity and reliability while addressing limitations in traditional methodologies. This highlights the significance of innovative methods in research, including adaptive designs, which are recognized for their capacity to optimize resource allocation and enhance the overall effectiveness of trials.
As the landscape of cancer treatment continues to evolve, the adoption of adaptive study designs is poised to play a pivotal role in shaping future research strategies and enhancing patient outcomes. Additionally, ongoing educational efforts, such as the EORTC course, emphasize the importance of staying updated on innovative methodologies in the field, which is particularly relevant for research directors striving to implement best practices in their studies.
Example 5: Clinical Trials Leading to Regulatory Changes
The clinical studies for the pioneering gene therapy aimed at inherited retinal diseases have catalyzed substantial regulatory transformations. The remarkable success of these experiments has led regulatory agencies to formulate new guidelines specifically for the approval of gene therapies. This evolution has not only expedited the pathway for groundbreaking treatments but has also established a benchmark for the assessment of similar innovative therapies in the future.
As of 2025, statistics indicate that the approval process for gene therapies has become significantly more efficient, with a marked increase in the number of therapies reaching the market. Notably, the FDA Adverse Event Reporting System (FAERS) is supported by over 150 countries, underscoring the global context of these regulatory changes. Furthermore, expert opinions emphasize that these regulatory changes are essential for fostering an environment conducive to innovation, ultimately enhancing treatment accessibility for individuals suffering from retinal diseases.
Gueddar highlights that in an industry already facing difficulties with patient enrollment and participation, organizations will need more adaptable and prompt payment procedures to enhance research outcomes. The implications of these advancements extend beyond retinal therapies, influencing regulatory practices across the Medtech landscape and paving the way for future innovations in gene therapy, as demonstrated by various Medtech clinical trial examples. Moreover, the incorporation of AI and automation in Regulatory Affairs is poised to streamline processes and enhance compliance, as emphasized in recent case analyses.
This technological evolution, coupled with major pharma companies' investments in antibody-drug conjugates (ADCs) for cancer therapy, illustrates a broader trend of innovation within the Medtech sector. At bioaccess, our extensive research study management services include feasibility assessments, site selection, compliance evaluations, study setup, import permits, project management, and reporting. These capabilities not only support the regulatory success of gene therapies but also contribute to job creation, economic growth, and healthcare improvement in local economies, particularly in Latin America.
By driving international collaboration and innovation in Medtech, we are transforming lives and enhancing the overall landscape of healthcare.
Example 6: Trials That Enhanced Device Efficacy
Recent clinical trials in Medtech focusing on the latest generation of orthopedic implants have showcased remarkable enhancements in patient outcomes compared to earlier models. These trials have yielded vital data regarding the long-term durability and effectiveness of these implants, leading to innovative designs that minimize complications and accelerate recovery times. For instance, research has indicated a mean change in the American Shoulder and Elbow Surgeons (ASES) score of 40.0 ± 24.2 following the implementation of advanced fixation techniques, underscoring significant improvements in functional outcomes.
Moreover, the exploration of new materials, such as pyrolytic carbon, shows promise in reducing wear and enhancing the longevity of implants. Preliminary findings indicate that these innovations may result in improved outcomes for individuals, although additional long-term evaluations are necessary to confirm their superiority over conventional materials like cobalt-chromium-molybdenum alloys and titanium. The materials utilized in shoulder implants have advanced considerably, with recent research suggesting that newer substances may enhance implant durability and patient results, but further long-term assessments are required.
The incorporation of hybrid designs, merging polyethylene pegs with metal posts, has also demonstrated favorable results. However, as highlighted in recent findings, long-term evaluations are needed to determine their superiority over other component types. As these advancements continue to progress, they are establishing new standards for orthopedic care, ultimately transforming the research landscape in this area, as evidenced by various Medtech clinical trial examples.
In this context, bioaccess® provides extensive management services essential for navigating the complexities of these investigations in Latin America. With expertise in viability assessments, location selection, compliance evaluations, experimental setup, import permits, project management, and reporting, bioaccess® ensures that clinical experiments are conducted efficiently and effectively. The company specializes in various study types, including Early-Feasibility Studies, First-In-Human Studies, Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF).
As Dipanwita Das, CEO & co-founder, emphasizes, 'Last, but certainly not least is regulatory preparedness. Regulations are becoming increasingly intricate, prescriptive, and demanding. Monitoring FDA guidance on innovative study designs and DCT methods while keeping up with global regulations ensures a smooth commercialization process, as well as data privacy. The ongoing commitment to regulatory preparedness and adherence to evolving standards, including compliance with INVIMA's regulatory functions, will be crucial in ensuring the successful commercialization of these innovative orthopedic devices.
Example 7: Lasting Impacts of Clinical Trials on Medtech Evolution
The transformative impact of Medtech clinical trial examples on the industry is particularly evident in the rapid evolution of minimally invasive surgical techniques (MIST). These evaluations have not only validated the efficacy of such techniques but have also provided valuable Medtech clinical trial examples that facilitate their widespread adoption across various medical fields. Consequently, individuals benefit from improved outcomes, including reduced postoperative pain, shorter recovery times, and lower healthcare costs.
A recent systematic review has shown that minimally invasive surgery is linked to less pain, a shorter hospital stay, and fewer complications. This evaluation also emphasized that MIST typically results in improved aesthetic outcomes and reduced morbidity, with 60% of studies indicating increased satisfaction scores among individuals.
Furthermore, the ongoing progress in outcomes from Medtech clinical trial examples underscores the significance of continuous research and innovation. The integration of minimally invasive techniques has been associated with significant enhancements in medical outcomes, as demonstrated by a systematic review published in 2023 by Carlos M. Ardila, Daniel Gonzalez-Arroyave, and Annie Marcela Vivares-Builes. This review focused on the efficacy of these methods in soft tissue management and determined that MIST is a practical option for handling soft tissues in dental procedures, further reinforcing its significance in improving patient care.
In addition to these advancements, extensive management services for research play a vital role in the success of Medtech projects. Services such as feasibility assessments, site selection, compliance reviews, experimental setup, import permits, project management, and reporting are crucial for ensuring that experiments are conducted efficiently and effectively. For instance, the recent presentation by Dr. Jorge Hernando Ulloa at the Charing Cross International Symposium showcased one-year data from the first-in-human VenoValve® study, highlighting advancements in vascular medicine and the importance of rigorous trial management.
As the Medtech landscape continues to evolve, the insights gained from Medtech clinical trial examples will play a crucial role in shaping future innovations. The lessons learned not only inform the development of new technologies but also ensure that care remains at the forefront of advancements in the industry. With the increasing adoption rates of minimally invasive techniques, the Medtech sector is poised for continued growth, driven by a commitment to improving patient outcomes through rigorous clinical research and international collaboration.
Conclusion
The exploration of clinical trials within the Medtech sector underscores their indispensable role in driving innovation and enhancing patient care. These trials constitute a critical framework for validating new medical technologies, ensuring compliance with safety and efficacy standards before they enter the market. By facilitating the transition from concept to practical application, clinical trials not only cultivate trust among stakeholders but also contribute to significant advancements in healthcare.
Throughout this article, various types of clinical trials—such as pivotal studies, early-feasibility studies, and post-market follow-up studies—have been highlighted for their unique contributions to the Medtech landscape. Each trial type plays a vital role in evaluating new devices under real-world conditions, ultimately leading to improved patient outcomes and more efficient regulatory processes. The case studies presented illustrate how successful trials have revolutionized treatments, including drug-eluting stents and minimally invasive surgical techniques, showcasing the transformative power of rigorous clinical research.
As the Medtech industry continues to evolve, the insights gleaned from these trials will shape future innovations and regulatory practices. The ongoing commitment to effective trial design and management, exemplified by organizations like bioaccess®, is crucial for fostering advancements that prioritize patient safety and care. The future of healthcare hinges on the successful execution of clinical trials, affirming their status as a cornerstone of Medtech innovation and a pathway to improved health outcomes for patients worldwide.
Frequently Asked Questions
What is the role of Medtech clinical trials in the development of medical technologies?
Medtech clinical trials are crucial for validating the safety and efficacy of medical technologies. They provide a structured framework for testing new devices, ensuring they meet regulatory criteria and deliver real benefits to healthcare.
How do Medtech clinical trials impact product development and stakeholder trust?
These trials enhance product development by facilitating the transition from concept to market and building trust among stakeholders, including healthcare providers and patients. They showcase the reliability of new medical devices through evidence-based results.
What challenges do Medtech companies face in research design?
Medtech companies encounter unique challenges that require customized research approaches. A key issue highlighted is the lower emphasis on simplifying enrollment in patient-support programs compared to biopharma participants, indicating a need for tailored strategies.
What benefits do risk-oriented methods bring to Medtech research?
Applying risk-oriented methods in research can improve data quality, increase resource efficiency, and shorten project timelines, ultimately accelerating the time to market for new devices.
What types of studies does bioaccess® manage in the Medtech sector?
Bioaccess® manages various types of studies, including Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Follow-Up Studies (PMCF).
How does bioaccess® support Medtech clinical trials in Latin America?
With over 20 years of experience, bioaccess® offers specialized expertise and adaptability to streamline the research process, significantly reducing the time and resources needed for successful completion of clinical trials.
What is the significance of Early-Feasibility Studies in Medtech?
Early-Feasibility Studies are essential for assessing the practicality and potential clinical utility of new technologies early in development, helping to identify challenges before larger-scale trials.
Why are Post-Market Follow-Up Studies important?
PMCF evaluations are critical for monitoring the long-term safety and effectiveness of medical devices after regulatory approval, ensuring compliance with standards and providing valuable data on real-world performance.
What upcoming regulatory changes are expected to impact Medtech clinical trials?
The FDA is expected to introduce the Single IRB Requirement in 2025, which aims to streamline the review process across various locations, enhancing the efficiency of key studies and accelerating the path to market for innovative devices.
How does the trend of sponsors insourcing data management processes affect the Medtech industry?
Insourcing data management processes allows sponsors to have enhanced control and transparency over research data, which is crucial for operational efficiency and improving data quality in the Medtech sector.

