Introduction
The Dominican Republic has rapidly positioned itself as a leading destination for medical device trials, attracting researchers with its robust healthcare infrastructure and diverse patient population. This burgeoning hub not only boasts modern facilities capable of supporting advanced clinical research but also benefits from a regulatory environment that facilitates efficient trial approvals. As the demand for innovative medical solutions grows, the Dominican Republic offers a unique combination of cost-effectiveness and streamlined processes, making it an appealing choice for medical device manufacturers.
With strategic collaborations and a commitment to ethical standards, the country is poised to enhance global health outcomes while driving economic growth through clinical research initiatives.
Why the Dominican Republic is a Prime Location for Medical Device Trials
The Dominican Republic has rapidly emerged as a premier destination for device trials, driven by several compelling factors. The country's healthcare infrastructure is robust, featuring modern hospitals and clinics equipped to support advanced clinical research. This infrastructure is complemented by a diverse patient population, offering researchers access to a wide array of demographics and medical conditions, which is crucial for comprehensive data collection. Moreover, the regulatory landscape in the Dominican Republic is favorable, with organizations like CONABIOS usually requiring an average of 45 days and up to 120 days for approvals, enabling a more efficient start of research.
Bioaccess® specializes in comprehensive trial management services, including:
- Feasibility assessments
- Site selection
- Compliance evaluations
- Trial setup
- Import permits
- Project management
- Reporting
With over 20 years of experience in Medtech, their expertise extends across various study types such as:
- Early-Feasibility
- First-In-Human
- Pilot
- Pivotal
- Post-Market Follow-Up Studies
This makes them a valuable partner for medical device manufacturers.
Recent developments emphasize the nation's dedication to improving medical research. A significant meeting between Cochrane's Editor-in-Chief and PAHO officials centered on promoting collaboration under a new memorandum of understanding, highlighting the strategic value of international partnerships in clinical research. Furthermore, despite facing challenges such as limited resources, regulatory complexities, and a misalignment of research priorities with the population's health needs, efforts to improve funding, training, and international collaboration are underway. The case study titled "Addressing Challenges in Clinical Research in the Dominican Republic" illustrates these issues and emphasizes the necessity of aligning research efforts with the healthcare needs of Dominicans.
Dr. Emily Messina encourages academics to leverage available resources, emphasizing the importance of adaptability in research methodologies. These combined factors make the Dominican Republic a highly appealing choice for medical device manufacturers seeking a cost-effective and timely site for their evaluations, while also contributing to job creation, economic growth, and improved healthcare outcomes in the region.
Navigating the Regulatory and Ethical Landscape for Clinical Trials
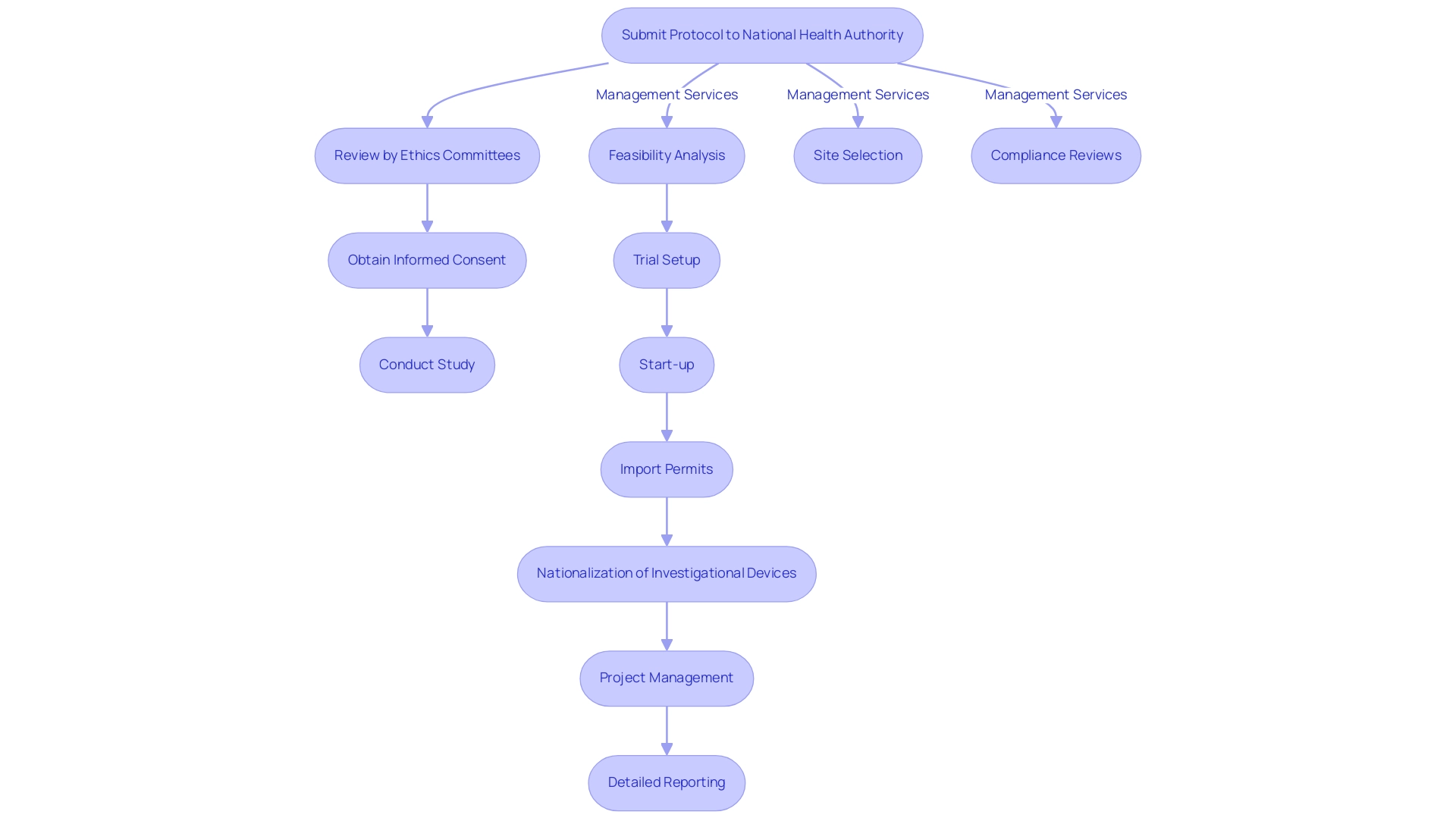
Conducting clinical studies in the Dominican Republic requires a thorough understanding of both regulatory and ethical considerations. The nation functions within a framework that corresponds with global standards, ensuring assessments are carried out ethically and responsibly. Researchers must submit their protocols to the National Health Authority for approval, which involves a rigorous review process by ethics committees and health ministries.
Furthermore, it is essential to obtain informed consent from all participants, ensuring they are fully aware of the study's purpose, procedures, and potential risks. Ethical committees play a critical role in overseeing studies, safeguarding participant rights and welfare.
To enhance the efficiency of your clinical trials, consider comprehensive clinical trial management services that encompass:
- Feasibility analysis
- Site selection
- Compliance reviews
- Trial setup
- Start-up
- Import permits
- Nationalization of investigational devices
- Project management
- Detailed reporting on research status, inventory, and both serious and non-serious adverse events
Experts such as Katherine Ruiz, an experienced Regulatory Affairs professional with extensive knowledge in devices and in vitro diagnostics in Colombia, can assist you in navigating these complexities. By effectively navigating these regulatory and ethical landscapes, researchers can ensure compliance and uphold the integrity of their studies.
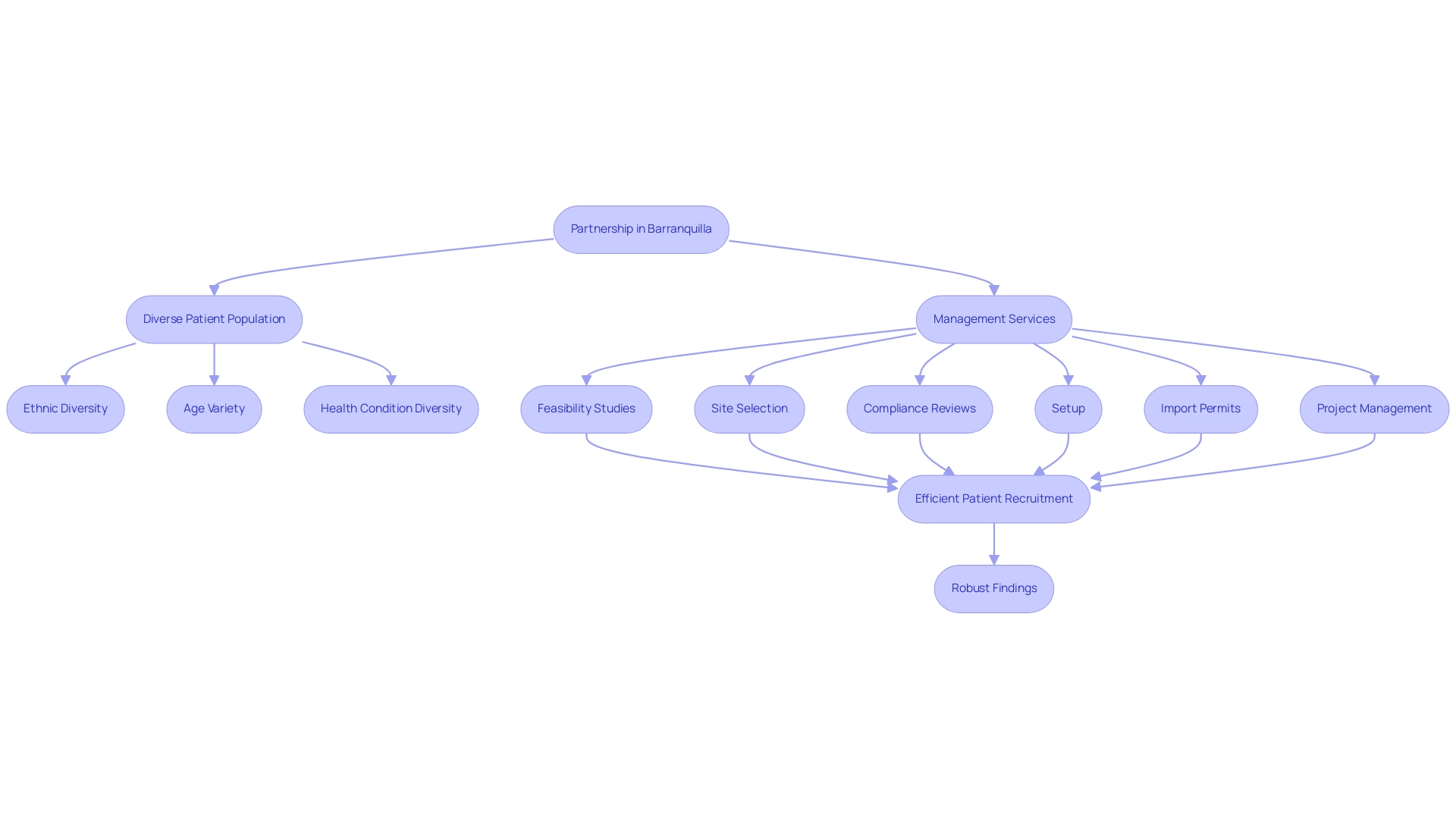
Access to a Diverse Patient Population
One of the standout benefits of conducting device evaluations in Colombia, particularly in Barranquilla, is the partnership between bioaccess™ and Caribbean Health Group, backed by Colombia's Minister of Health, Juan Pablo Uribe. This partnership is establishing Barranquilla as a premier location for medical research in Latin America, utilizing a varied patient demographic that includes a mix of ethnicities, ages, and health conditions. Such diversity allows researchers to gather data that reflects a wide range of patient experiences, which is especially beneficial for assessing the efficacy and safety of medical devices across different demographic groups.
Moreover, with GlobalCare Clinical Trials achieving over a 50% reduction in recruitment time and a 95% retention rate, the active collaboration between healthcare providers and researchers not only facilitates efficient patient recruitment but also enhances the robustness of findings.
By leveraging this diverse patient base and the extensive management services provided by bioaccess®, which encompass:
- feasibility studies
- site selection
- compliance reviews
- setup
- import permits
- project management
- reporting
researchers can effectively address the needs of various populations. This partnership encourages economic development and healthcare enhancement in the area, generating employment and advancing international acknowledgment of Colombia's research capabilities.
Cost-Effectiveness of Conducting Trials
Conducting medical device studies in the Dominican Republic provides significant cost advantages compared to other regions. The overall expenses associated with research trials—including participant recruitment, site fees, and operational costs—tend to be lower in the Dominican Republic. This cost-effectiveness is largely attributed to the favorable exchange rate and lower labor costs, which allow sponsors to allocate resources more efficiently.
Furthermore, bioaccess® offers extensive management services for research projects, including:
- Feasibility assessments
- Site selection
- Compliance evaluations
- Project setup
These services simplify the process. Our expertise encompasses various types of studies, such as:
- Early-Feasibility Studies (EFS)
- First-In-Human Studies (FIH)
The accelerated regulatory pathways further reduce the time to market for new devices, enhancing the financial benefits. With over 20 years of experience in Medtech, our proven team ensures high-quality results while optimizing budgets. By selecting the Dominican Republic for research studies, sponsors can accomplish their goals supported by our vast knowledge.

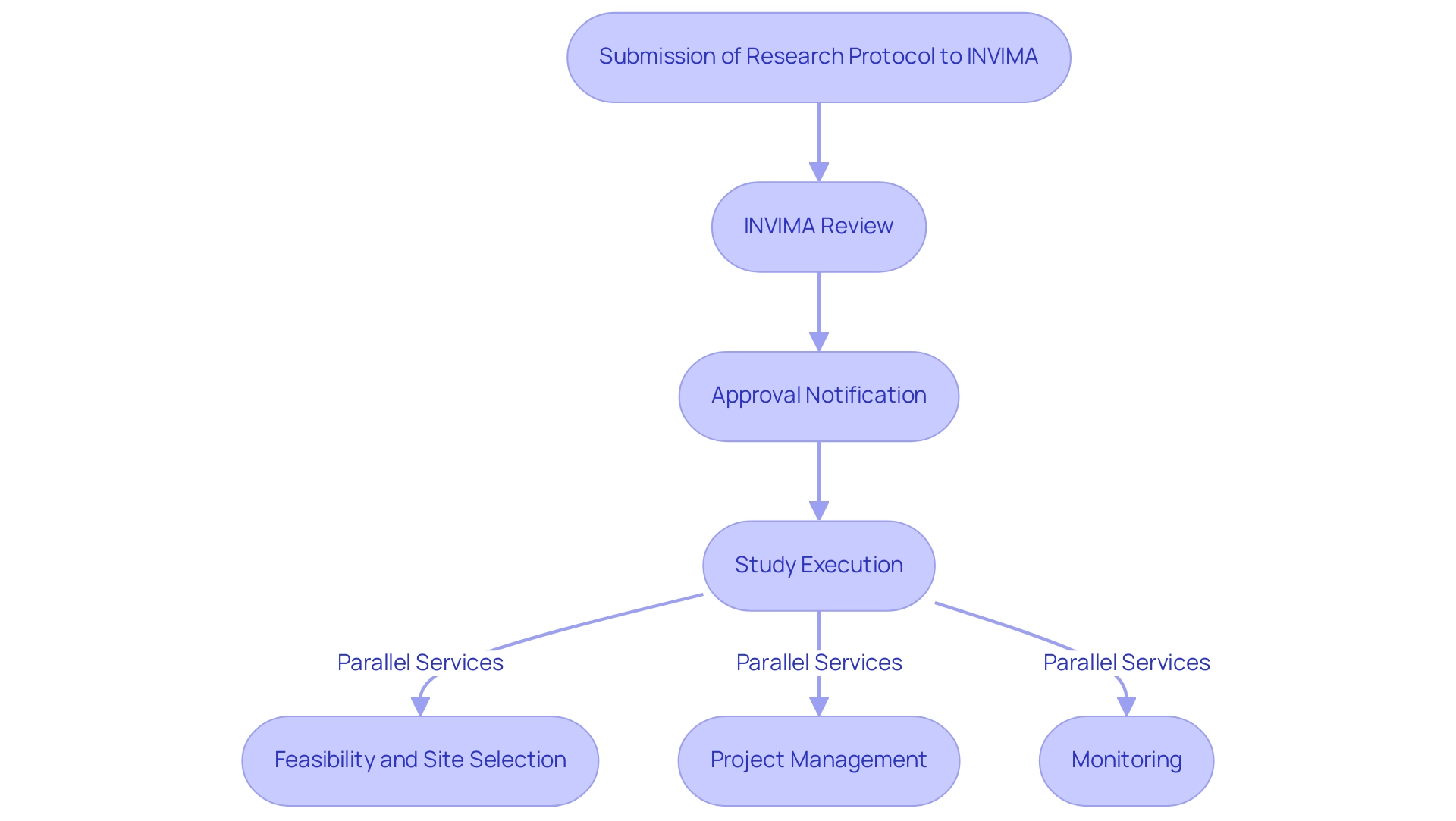
Streamlined Approval Processes
Colombia is acknowledged for its efficient regulatory framework that enables the swift commencement of research studies, making it an appealing choice for investigators. The Colombia National Food and Drug Surveillance Institute (INVIMA) supervises this process, ensuring adherence to health standards while accelerating the examination of research protocols.
With over 20 years of experience in Medtech, bioaccess® provides expertise in managing clinical studies—including:
- Early-Feasibility
- First-In-Human
- Pilot
- Pivotal
- Post-Market Follow-Up Studies
This enables sponsors to expect significantly shorter timelines for obtaining necessary approvals. Our comprehensive service capabilities include:
- Feasibility and selection of research sites
- Project management
- Monitoring
This allows researchers to focus on executing their trials effectively. This streamlined approach generates valuable data that propels the advancement of healthcare knowledge and accelerates the introduction of innovative devices to the market. Additionally, bioaccess® is vetted and approved as a business service provider for U.S. device companies, further enhancing our credibility and relevance in the industry.
Strong Collaboration with Local Institutions
The effectiveness of medical device studies in Barranquilla is significantly enhanced through strategic collaboration with local healthcare institutions, such as Caribbean Health Group, and support from Colombia's Minister of Health. This collaboration, revealed on March 29, 2019, seeks to establish Barranquilla as a prominent location for medical research in Latin America.
By leveraging bioaccess™'s extensive network and resources, researchers gain access to state-of-the-art facilities, experienced professionals, and a diverse pool of potential study participants. Significantly, GlobalCare Clinical Studies has collaborated with bioaccess™ to improve ambulatory services in Colombia, achieving over a 50% decrease in recruitment time and an impressive 95% retention rate for participants.
The leadership of Julio Martinez-Clark, CEO of bioaccess™, emphasizes the significance of operationalizing clinical studies and mentoring startups to foster regional opportunities in Medtech. Alongside him, Monica Mora, the Chief Operating Officer, specializes in streamlining operations and regulatory strategies for medical device companies entering the Latin American market, implementing comprehensive processes to navigate local regulations effectively.
Such collaborations not only enhance the credibility of studies but also align with local regulations, ensuring high-quality, well-managed research that effectively addresses urgent health challenges in the region.
Commitment to Patient Safety and Ethical Standards
In the Dominican Republic, there is a strong commitment to patient safety and adherence to ethical standards in clinical research. Researchers must adhere to strict protocols that emphasize participant welfare, including:
- Acquiring informed consent
- Guaranteeing that participants are completely aware of the risks and benefits linked to the study
Ethical review boards play a crucial role in overseeing research, ensuring that they meet the highest ethical standards. This commitment to patient safety not only protects participants but also enhances the credibility of the research conducted in the Dominican Republic, making it an appealing choice for sponsors and researchers alike.
Alongside the comprehensive trial management services provided by bioaccess®, which encompass:
- Feasibility studies
- Site selection
- Compliance reviews
- Trial setup
- Strong project management
the region emerges as a leading center for Medtech innovation. Bioaccess® ensures regulatory excellence through meticulous site selection, thorough reviews, and strong project management practices, driving global health improvement through international collaboration and innovative clinical practices.
Conclusion
The Dominican Republic has emerged as a premier destination for medical device trials, driven by its robust healthcare infrastructure, diverse patient population, and a regulatory environment conducive to efficient study approvals. The integration of comprehensive clinical trial management services offered by experienced organizations such as Bioaccess® enhances the ability of researchers to navigate the complexities of trial execution. This strategic combination not only facilitates timely access to valuable data but also addresses the urgent healthcare needs of the local population.
Moreover, the country’s commitment to ethical standards and patient safety underscores its dedication to conducting responsible clinical research. By ensuring rigorous protocols and fostering strong collaborations with local institutions, the Dominican Republic is positioning itself as a leader in the field of Medtech. These efforts contribute significantly to economic growth and the advancement of healthcare outcomes, making the region an attractive option for medical device manufacturers seeking cost-effective trial locations.
In summary, the Dominican Republic stands out as a unique hub for clinical research, offering a blend of efficiency, ethical integrity, and diverse demographics. As the global demand for innovative medical solutions continues to rise, the opportunities presented by conducting trials in this region will likely expand, benefiting both researchers and the communities they serve.




