Overview
The article "Top 7 Clinical Research Challenges in Latin America: Insights You Must Know" addresses the significant hurdles faced in clinical research within the region, including:
- Regulatory complexities
- Patient recruitment difficulties
- Logistical issues
It highlights that, despite increased funding and evolving partnerships aimed at improving the research environment, challenges such as:
- Inadequate infrastructure
- Cultural barriers
continue to impede progress. These persistent obstacles necessitate targeted strategies to enhance study efficiency and patient engagement.
Introduction
The landscape of clinical research in Latin America is both promising and complex, teeming with challenges that require innovative solutions and strategic partnerships. With investment in the sector skyrocketing—from a mere $3-4 million to over $50 million annually in the Andean Region—stakeholders are increasingly recognizing the potential for growth and improvement. However, navigating intricate regulatory frameworks, logistical hurdles, and recruitment difficulties remains a significant barrier to success.
By fostering collaborations with local healthcare providers and leveraging the region's diverse patient demographics, organizations can enhance their clinical trial strategies. This article delves into the multifaceted challenges and opportunities within Latin America's clinical research environment, highlighting key trends and the critical importance of building strong local partnerships to drive advancements in healthcare.
Overview of Clinical Research Challenges in Latin America
The distinctive array of challenges faced in clinical investigations underscores the clinical research hurdles in Latin America, which can significantly impede the advancement of studies. Key obstacles encompass intricate regulatory frameworks, difficulties in patient recruitment, logistical hurdles, and operational inefficiencies. Notably, the annual funding in the health study sector in the Andean Region has surged from $3-4 million to over $50 million, reflecting a growing awareness of the necessity for enhanced investigative capabilities.
However, this increase in funding must be accompanied by improvements in the quality of investigative practices. Elevating financial resources in science and technology is crucial for enhancing the overall caliber of studies in the region.
In this context, bioaccess™ has partnered with the Caribbean Health Group to position Barranquilla as a prominent site for medical evaluations, bolstered by support from Colombia's Minister of Health. This collaboration was unveiled during a gathering in Miami, FL, on March 29, 2019, at PROCOLOMBIA's office, where the Minister openly endorsed the initiative aimed at improving medical studies in Colombia. The partnership seeks to streamline medical research studies and underscores the commitment to enhancing the research environment in the area.
Furthermore, GlobalCare Clinical Trials has also allied with bioaccess™ to elevate trial ambulatory services in Colombia, achieving over a 50% reduction in recruitment time and 95% retention rates. This demonstrates the tangible benefits of such partnerships.
The region's diverse patient demographics present both challenges and opportunities for trials. Researchers’ recruitment efforts are complicated by the clinical research challenges in Latin America, which include varying health conditions, cultural perceptions, and access to healthcare. Additionally, the evolving oversight landscape necessitates that stakeholders remain agile and informed.
As industry authority Gustavo Gossling observes, 'The approval process is an increasingly vital aspect in Brazil,' highlighting the necessity of addressing regulatory challenges in this significant market.
A recent survey aimed at oncology doctors revealed substantial obstacles to conducting medical studies, including a lack of knowledge, insufficient compensation, and institutional apathy. These factors result in reduced patient involvement rates in research studies, emphasizing the need for targeted approaches to engage both healthcare providers and patients. The survey, titled 'Oncology Physicians' Perspectives on Research,' illustrates these barriers and stresses the importance of addressing them to enhance patient participation in trials.
As the research environment in Latin America continues to evolve, grasping the clinical research challenges in the region is essential for stakeholders aiming to navigate the landscape successfully. By adapting strategies—including comprehensive research management services such as feasibility studies, site selection, compliance reviews, study setup, import permits, project management, and reporting—researchers can better position themselves to capitalize on the unique opportunities that the region offers. Specifically, bioaccess™ provides a complete range of services to ensure compliance and efficiency throughout the research study process.
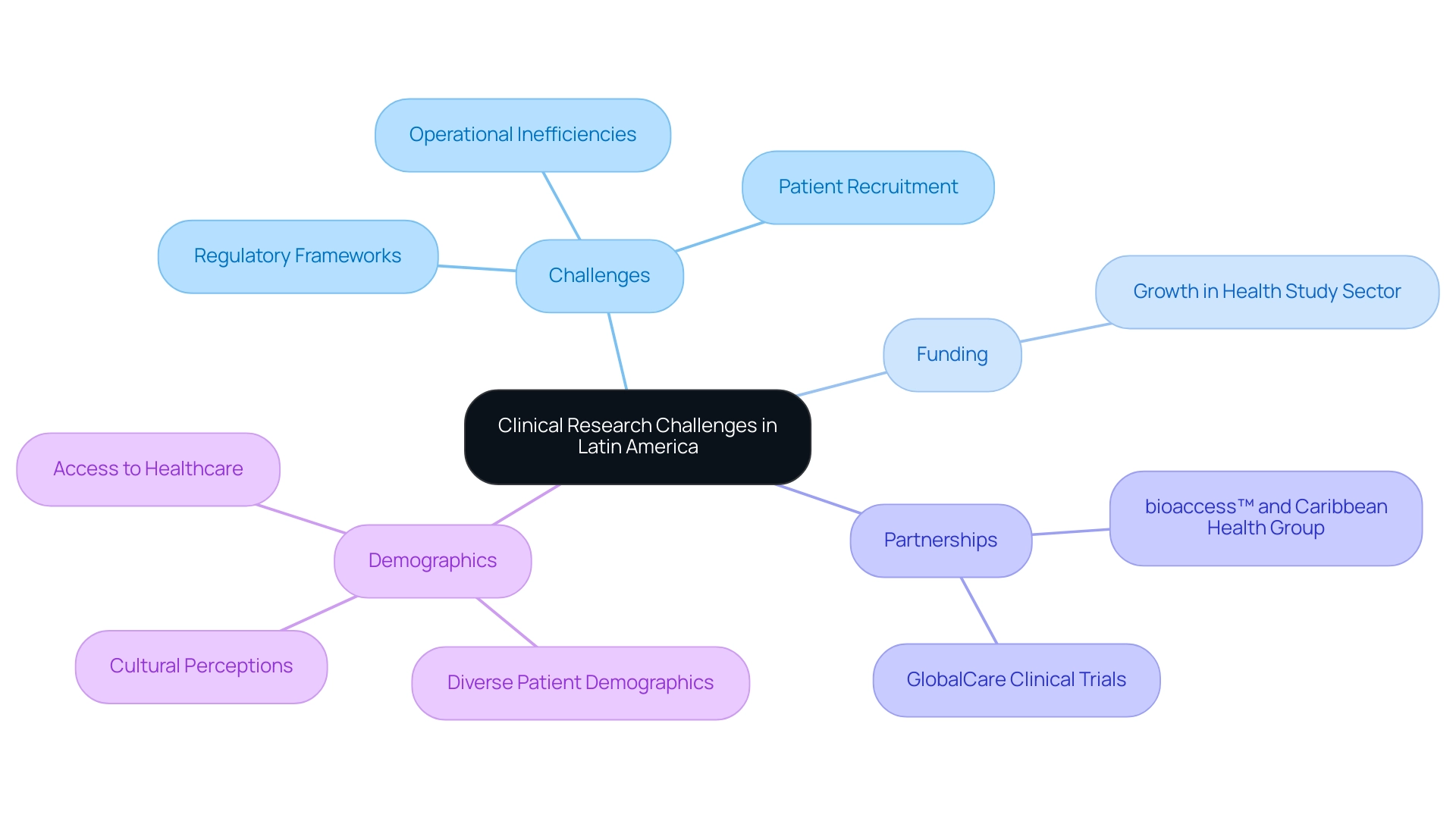
Recruitment Difficulties: Finding the Right Participants
Recruiting participants for clinical research studies in Latin America presents unique challenges influenced by cultural differences, language barriers, and a general lack of awareness regarding medical research. Many prospective participants may not fully grasp the purpose and advantages of research studies, leading to reluctance about their involvement. Additionally, socioeconomic factors often limit access, highlighting the necessity for targeted outreach and education to facilitate successful recruitment.
To tackle these challenges, employing strategies such as community engagement and culturally sensitive communication is essential. Utilizing local community leaders to disseminate information can build trust and enhance comprehension of research studies, aligning with bioaccess's commitment to enabling effective recruitment. Furthermore, leveraging technology to streamline the recruitment process can help alleviate logistical barriers that often deter underrepresented groups from participating. A case study on diversity in medical study recruitment underscores that addressing these logistical issues can significantly boost participation rates among diverse communities.
Statistics reveal that the annual investment in the medical study sector in Latin America’s Andean Region has surged from $3-4 million to over $50 million, indicating a growing recognition of the region's potential for medical exploration. This increase reflects the rising interest in conducting experiments and stresses the importance of developing effective recruitment strategies tailored to the local context. As Julio G. Martinez-Clark, CEO, noted, 'Colombia has acknowledged these advantages and has an ambitious science, technology, and innovation strategy for 2022–2031 to transform into a knowledge economy,' emphasizing the regional commitment to advancing medical studies.
Moreover, the prospects for conducting vaccine studies in Latin America, bolstered by the availability of bilingual U.S. board-certified doctors and significantly reduced costs for studies, further illustrate the benefits of undertaking experiments in the region. By understanding and addressing the cultural obstacles and recruitment challenges unique to Latin America, research organizations like bioaccess® can leverage their expertise in feasibility studies, site selection, study preparation, and project management to tackle the clinical research challenges in Latin America and enhance participant recruitment initiatives. Additionally, the economic impact of medical studies on local economies, including job creation and healthcare improvement, is substantial.
By fostering global cooperation and innovation in Medtech, bioaccess® is poised to contribute to more representative and effective trials.
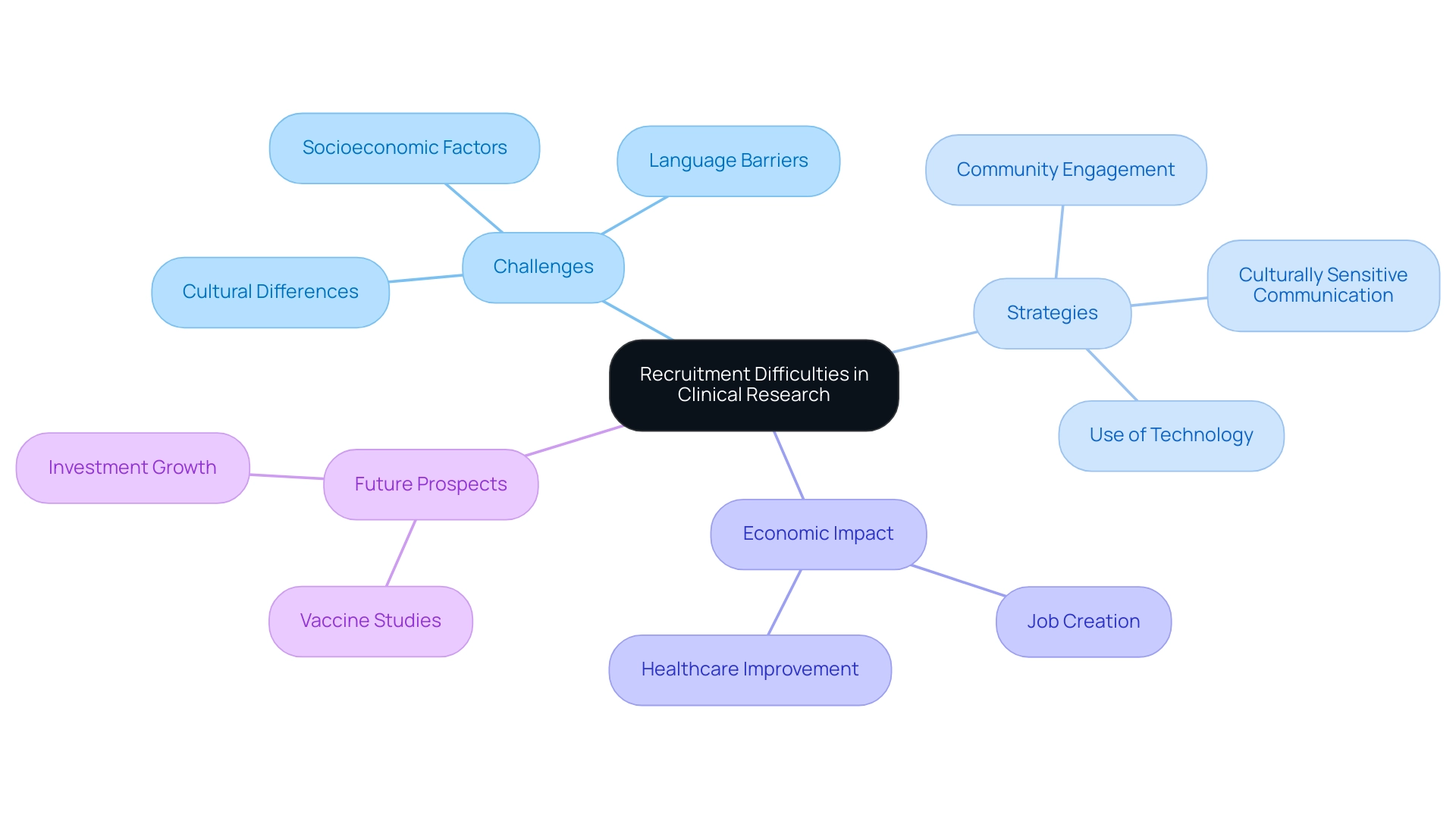
Navigating Regulatory Hurdles in Clinical Trials
The oversight environment in Latin America significantly contributes to the clinical research challenges faced by research professionals, characterized by substantial differences among nations. Lengthy approval processes represent a common hurdle, often extending timelines and inflating costs. Notably, recent statistics reveal that conducting medical research in Colombia can yield savings exceeding 30% compared to North and Western Europe, underscoring the potential for economic studies in the region despite the existing legal complexities.
bioaccess offers a comprehensive suite of research management services, encompassing:
- feasibility studies
- site selection
- compliance reviews
- setup
- import permits
- project management
- detailed reporting on study status and adverse events
Importantly, our services include thorough review and feedback on study documents to ensure compliance with country requirements and the nationalization of investigational devices, which are crucial for navigating the complexities imposed by INVIMA, Colombia's National Food and Drug Surveillance Institute, recognized as a Level 4 health authority by PAHO/WHO.
In 2024, Brazil achieved a significant milestone with the endorsement of Law 14.874/24, aimed at streamlining the evaluation process for trials. This legislation is crafted to reduce bureaucratic barriers and enhance predictability in regulatory approvals, which could greatly benefit both researchers and patients by facilitating quicker access to innovative treatments, thereby addressing the clinical research challenges in Latin America. Additionally, pending legislation (PL 7082/2017) in Brazil seeks to further tackle these challenges by establishing clearer regulations for the timely approval of study protocols.
Should this legislation be enacted, it could revolutionize the clinical research landscape, particularly in oncology, by improving access to new cancer treatments.
As Katherine Ruiz, an expert in compliance matters for medical devices and in vitro diagnostics in Colombia, notes, navigating these hurdles necessitates vigilance and proactive engagement with local authorities during the planning phases of studies. This strategy not only aids in compliance but also fosters a smoother pathway to successful initiation and execution. Dushyanth Surakanti, Founder and CEO of Sparta Biomedical, emphasizes, 'Our dedication to regulatory excellence and innovation in medtech research is essential for progressing medical studies in the region.
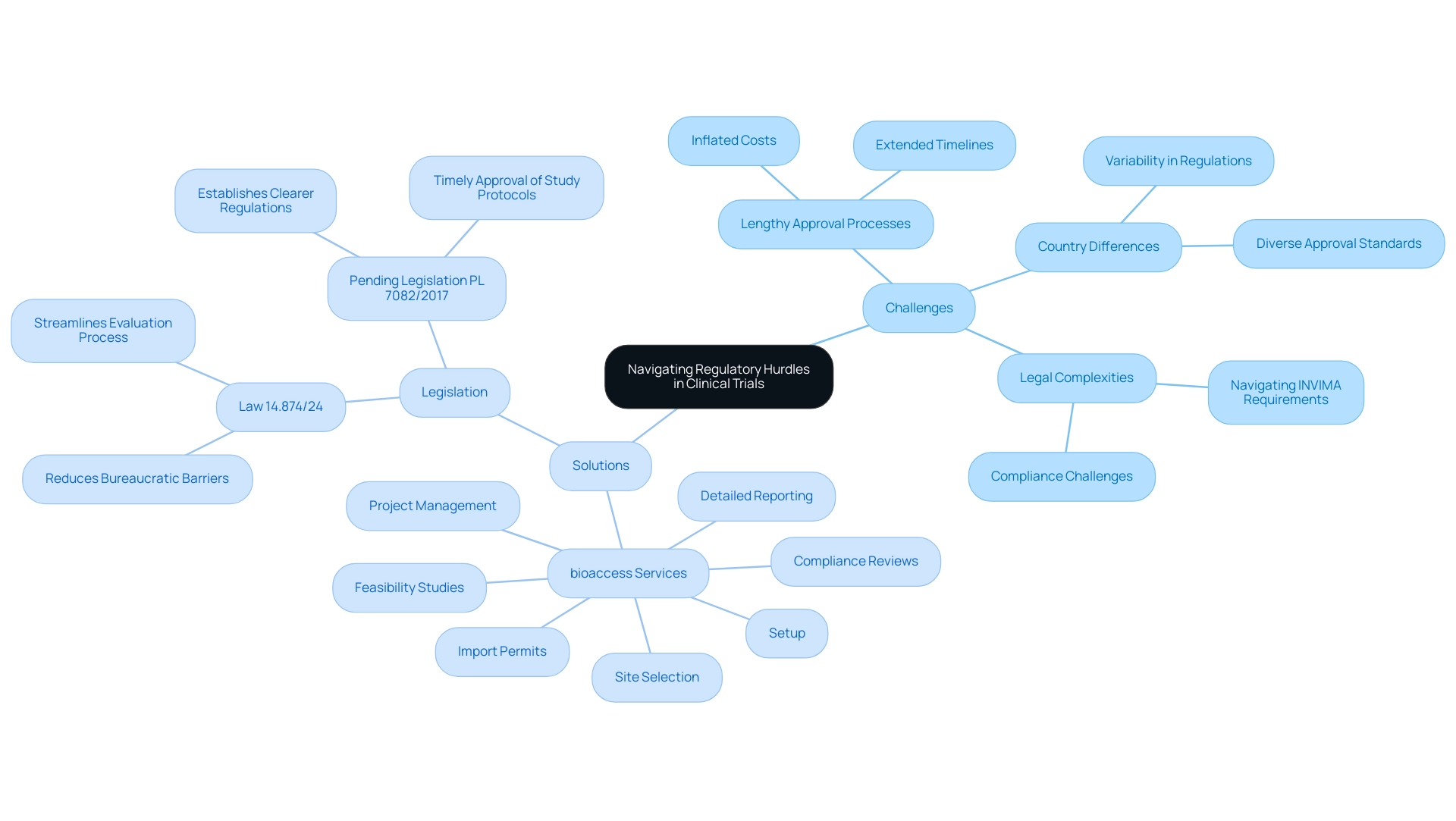
Logistical Complexities: Managing Clinical Trial Supplies
Overseeing clinical research challenges in Latin America presents numerous logistical difficulties, including strict import regulations, transportation obstacles, and potential supply chain interruptions. These factors can significantly affect timelines and inflate costs, making effective management crucial for overcoming clinical research challenges in the region. Notably, Colombia emerges as a premier destination for first-in-human (FIH) studies, boasting significant competitive advantages such as over 30% cost savings compared to North America and Western Europe, swift approvals averaging 90-120 days, and a highly ranked healthcare system.
Furthermore, Colombia offers substantial R&D tax incentives, including a 100% tax deduction for investments in science, technology, and innovation projects, further enhancing its appeal for clinical studies. To effectively tackle the clinical research challenges in Latin America, organizations must devise robust supply chain strategies tailored to local conditions and regulatory frameworks.
Collaboration with local suppliers and logistics providers is essential for overcoming these challenges by enhancing operational efficiency and ensuring the timely delivery of critical materials. As Dushyanth Surakanti, Founder and CEO of Sparta Biomedical, observed regarding his experience with bioaccess during its initial human assessment in Colombia, leveraging local expertise can streamline processes and mitigate risks. The medical research supply and logistics sector in Latin America is projected to expand from USD 295.8 million in 2023 to USD 472.6 million by 2030, reflecting a compound annual growth rate (CAGR) of 6.9% from 2024 to 2030, despite the ongoing challenges in clinical research.
The clinical research challenges in Latin America are influenced by diverse patient populations, improved access to participants due to universal healthcare coverage that encompasses about 95% of the Colombian population, and recent regulatory reforms aimed at expediting trial application approvals.
Expert opinions underscore the importance of clear communication in navigating the complexities of research studies. As Saisuman Revankar articulates, 'Clear communication is critical for navigating the complexities of research studies, ensuring that all parties remain aligned.' By applying efficient logistics approaches and utilizing local knowledge, organizations can mitigate risks associated with supply chain interruptions and enhance the overall success of addressing clinical research challenges in Latin America.
Moreover, employing local knowledge and customized strategies is vital for addressing the unique clinical research challenges in the region, reinforcing the necessity for collaboration with local suppliers and logistics providers.
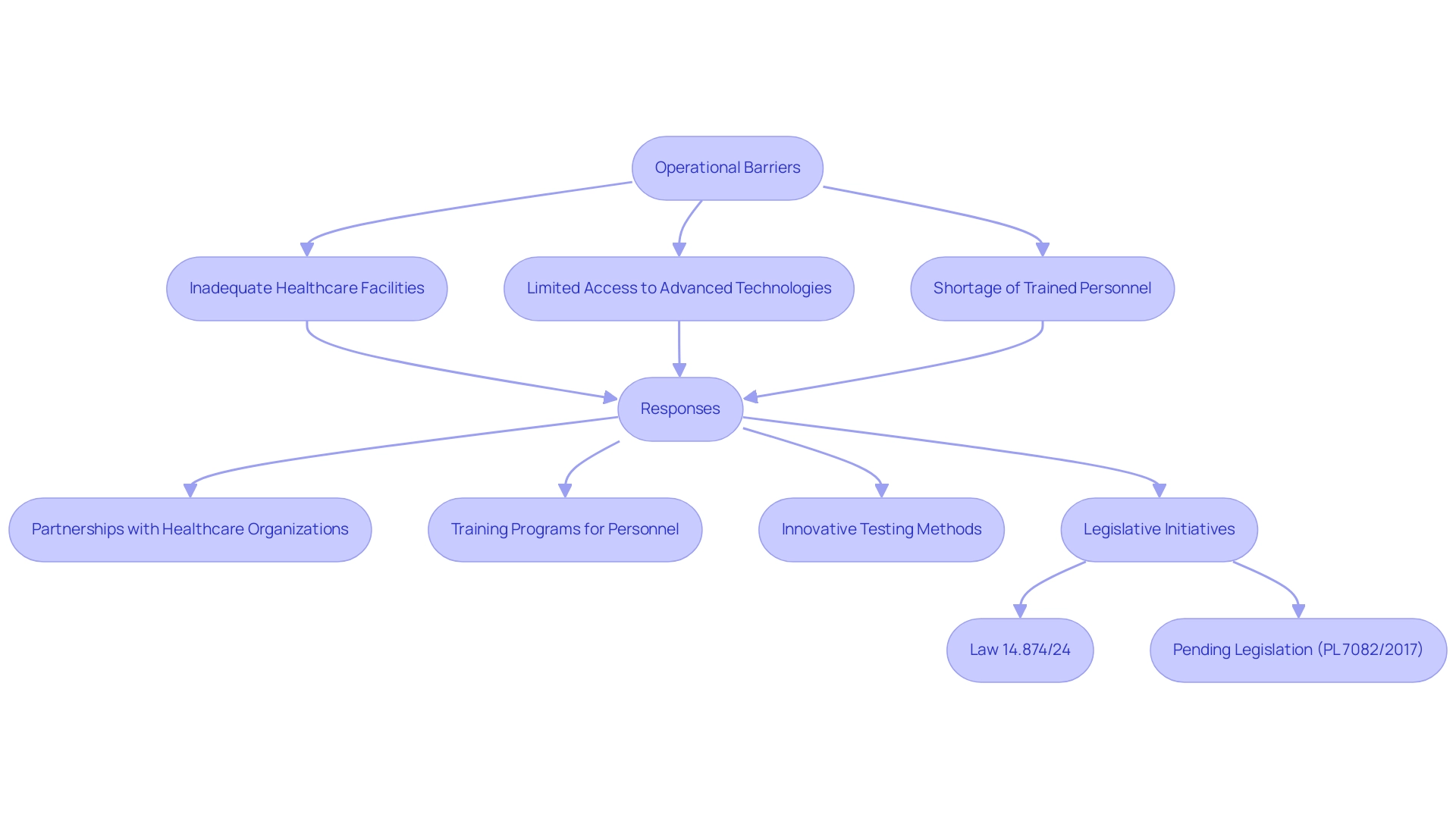
Operational Barriers: Overcoming Infrastructure Limitations
Infrastructure constraints in Latin America present significant challenges for clinical research, greatly impacting the implementation of health evaluations. Key issues include:
- Inadequate healthcare facilities
- Limited access to advanced medical technologies
- A shortage of trained personnel
These factors contribute to a disproportionately low availability of early-phase research studies in the region, highlighting the clinical research challenges in Latin America, despite its substantial population.
As Mirella Nardo from ICESP emphasizes, 'Despite its substantial population, the disproportionately low availability of early-phase research studies illustrates the clinical research challenges in Latin America and underscores the urgent need for increased research efforts.' In response to these challenges, Julio Martinez-Clark, CEO of bioaccess, has championed initiatives to implement medical studies, viewing the region as an untapped market for Medtech innovation. His leadership encompasses mentoring startups at Macondo Labs and advising Colombian government agencies on positioning the country as a prime location for medical research.
Partnerships with regional healthcare organizations, such as the Caribbean Health Group, aim to establish Barranquilla as a leading location for clinical studies, while also addressing the clinical research challenges in Latin America. The support of Colombia's Minister of Health, who advocates for improved clinical investigations, is pivotal in fostering trust and ensuring the success of these initiatives. This effort is critical for enhancing capacity and facilitating access to necessary resources. Furthermore, investing in extensive training programs for study personnel is essential to equip them with the skills needed to conduct high-quality experiments.
Innovative methods, such as decentralized testing models, can significantly mitigate infrastructure limitations. By connecting studies more directly with patients, these models enhance participation rates and simplify the testing process.
Recent legislative initiatives, such as Law 14.874/24, approved in May 2024, aim to optimize the evaluation process for medical studies in Brazil, addressing the clinical research challenges in Latin America and reflecting an increasing acknowledgment of the necessity for better infrastructure in medical inquiries. Additionally, pending legislation (PL 7082/2017) seeks to improve research practices by establishing maximum timelines for the approval of research protocols, which could lead to better access to new drugs and enhanced research development in Brazil.
By promoting ethical partnerships and employing innovative strategies, stakeholders can foster trust and ensure the success of research initiatives in the area, ultimately aiding the advancement of medical devices that can greatly benefit individuals' lives.
Building Partnerships: Collaborating with Local Stakeholders
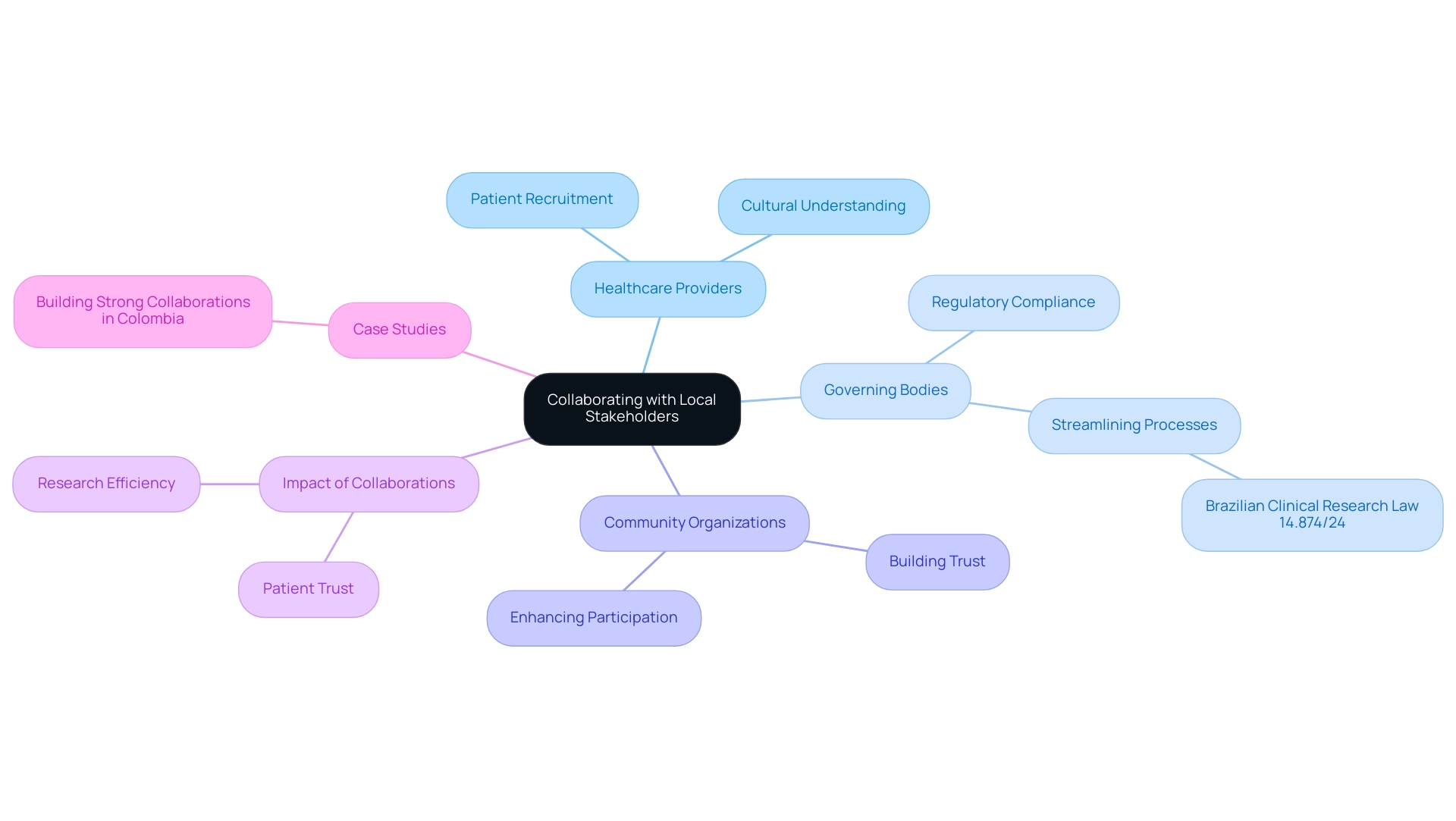
Forming strong collaborations with local stakeholders—such as healthcare providers, governing bodies, and community organizations—is essential for effectively addressing clinical research challenges in Latin America. These partnerships not only simplify patient recruitment but also bolster compliance with regulations and enhance overall study efficiency. By engaging local experts, researchers can leverage their profound understanding of the healthcare landscape, which is crucial for navigating cultural nuances and fostering trust within communities.
This trust is vital for increasing patient involvement, especially considering that knowledge of research options remains limited, with 85 percent of patients unaware of their participation opportunities.
The Brazilian Clinical Research Law 14.874/24 exemplifies the region's commitment to improving research processes by reducing regulatory timelines, thereby facilitating quicker access to innovative treatments. Furthermore, the growing number of skilled researchers in Latin America signals a promising expansion of the region's capacity to tackle clinical research challenges. Successful case studies, particularly those showcasing partnerships in Colombia, demonstrate how robust collaborations among stakeholders can enhance patient trust and participation in studies.
Dushyanth Surakanti, Founder and CEO of Sparta Biomedical, underscores this notion, asserting that "the organization’s dedication to regulatory excellence and innovation in medtech development" is crucial for advancing medical studies. He highlights that his experience with bioaccess during its inaugural human study in Colombia illustrated the significance of these partnerships in achieving successful outcomes.
Moreover, media coverage from sources such as Clinical Leader underscores the importance of medical studies in Colombia, showcasing the region's potential as a competitive site for clinical trials. Articles from Clinical Leader emphasize both the advancements and challenges within trials, further reinforcing the narrative of growth in the region. These collaborations not only position Latin America favorably within the global scientific landscape but also address clinical research challenges, paving the way for progress in genomics and personalized medicine.
Additionally, understanding the role of INVIMA, the Colombia National Food and Drug Surveillance Institute, is essential, as it oversees regulatory functions and medical device oversight, classifying Colombia as a Level 4 health authority by PAHO/WHO.
Future Trends: Opportunities for Growth in Clinical Research
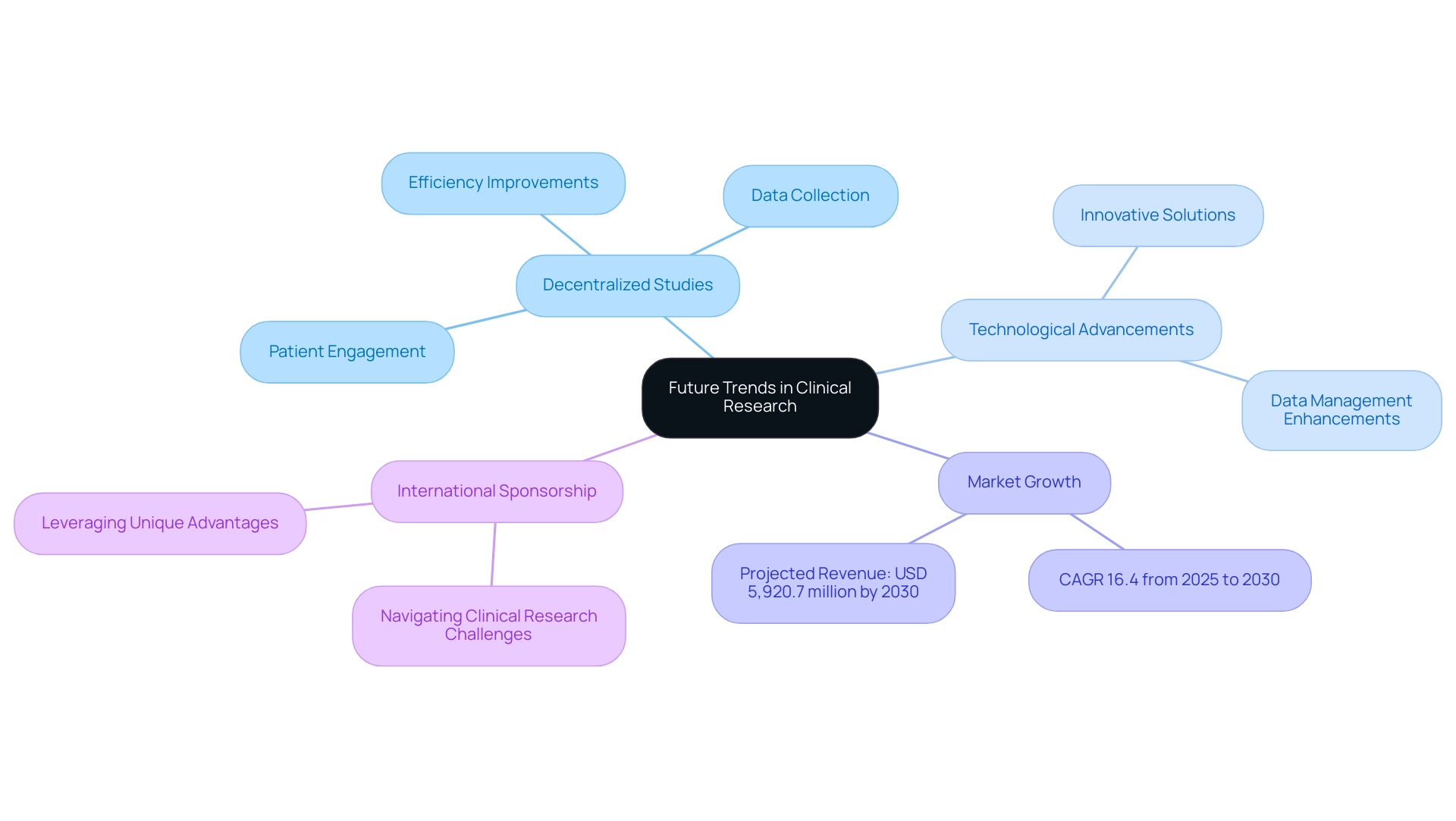
The medical investigation environment in Latin America is undergoing considerable change, presenting significant opportunities for expansion in clinical research. A notable trend is the increasing adoption of decentralized clinical studies, leveraging technology to enhance patient engagement and streamline data collection. This approach not only improves the effectiveness of experiments but also aligns with the growing focus on patient-centered techniques, ensuring studies are more accessible and relevant to diverse populations.
For instance, 45% of Alcon's data is entered on the same day as the visit date, showcasing the efficiency improvements in data management within decentralized studies.
Advancements in technology are further reshaping the research landscape, with innovative solutions driving enhancements in experimentation processes and data management. The medical research technology and services market in Latin America is projected to reach an impressive USD 5,920.7 million by 2030, reflecting a compound annual growth rate (CAGR) of 16.4% from 2025 to 2030. This growth is fueled by the rising demand for effective testing procedures and improvements in research infrastructure, positioning the region as a key player in the global market.
As noted by the Head of Clinical Data Engineering, "Traditionally, data management was outsourced to our CRO vendor partners. Part of the initiative is to bring all our studies in-house so that our internal teams can start working on it. They can be more hands-on, and we operationalize studies in-house and we are able to take control of our data, and we deliver for our patients with high quality."
Moreover, the region's diverse patient populations and evolving regulatory frameworks are attracting more international sponsors, eager to navigate the clinical research challenges in Latin America while leveraging the unique advantages the area offers. Comprehensive research management services—including:
- Early-Feasibility Studies (EFS)
- First-In-Human Studies (FIH)
- Pilot Studies
- Pivotal Studies
- Post-Market Follow-Up Studies (PMCF)
Along with feasibility studies, site selection, compliance reviews, setup, import permits, project management, and reporting, are essential for navigating this evolving landscape. Implementing risk-based approaches in research trials can lead to higher data quality, greater resource efficiency, and shorter study timelines, ultimately accelerating time to market.
By embracing these trends and adapting their strategies, organizations can effectively navigate the clinical research challenges in Latin America and capitalize on the opportunities presented by the ongoing evolution of the sector.
Conclusion
The clinical research landscape in Latin America presents both formidable challenges and significant opportunities. As emphasized throughout this article, navigating intricate regulatory frameworks, logistical hurdles, and recruitment difficulties is essential for stakeholders striving to enhance their clinical trial strategies. The remarkable surge in investment—from $3-4 million to over $50 million annually in the Andean Region—reflects a growing acknowledgment of the region's potential. However, it is evident that this increase in funding must be paralleled by improvements in research quality and operational efficiency.
Partnerships with local healthcare providers and community organizations are critical for surmounting these challenges. By cultivating trust and understanding within diverse patient populations, organizations can enhance their recruitment efforts and ultimately boost patient participation rates. Successful collaborations, such as those between bioaccess™ and local stakeholders, illustrate how leveraging local expertise can streamline processes and mitigate risks, thereby enhancing the overall success of clinical trials.
Looking ahead, the embrace of innovative methodologies, including decentralized clinical trials and advancements in technology, positions Latin America as a formidable contender in the global clinical research arena. By adopting these trends and refining strategies to align with local contexts, stakeholders can harness the unique advantages that the region offers. As the landscape continues to evolve, the commitment to forging robust local partnerships and addressing multifaceted challenges will be pivotal in driving advancements in healthcare and improving patient outcomes across Latin America.
Frequently Asked Questions
What are the main challenges faced in clinical investigations in Latin America?
The main challenges include intricate regulatory frameworks, difficulties in patient recruitment, logistical hurdles, and operational inefficiencies.
How has funding in the health study sector changed in the Andean Region?
Annual funding in the health study sector has increased from $3-4 million to over $50 million, indicating a growing awareness of the need for enhanced investigative capabilities.
What initiatives have been taken to improve medical studies in Colombia?
Bioaccess™ has partnered with the Caribbean Health Group to enhance medical evaluations in Barranquilla, supported by Colombia's Minister of Health. This partnership aims to streamline medical research studies.
What impact have partnerships like the one between GlobalCare Clinical Trials and bioaccess™ had on trial services in Colombia?
The partnership has resulted in over a 50% reduction in recruitment time and achieved 95% retention rates for trial ambulatory services.
What factors complicate patient recruitment in Latin America?
Recruitment is complicated by varying health conditions, cultural perceptions, access to healthcare, and a general lack of awareness regarding medical research.
What are the barriers to conducting medical studies identified in a recent survey of oncology doctors?
Barriers include a lack of knowledge, insufficient compensation, and institutional apathy, which lead to reduced patient involvement in research studies.
How can researchers improve patient recruitment in Latin America?
Employing strategies such as community engagement, culturally sensitive communication, and leveraging technology can facilitate successful recruitment.
What is the significance of the increase in investment in the medical study sector in Latin America?
The increase in investment reflects a growing recognition of the region's potential for medical exploration and emphasizes the importance of effective recruitment strategies tailored to the local context.
What economic impacts do medical studies have on local economies in Latin America?
Medical studies contribute to job creation and improvements in healthcare, highlighting their substantial economic impact on local communities.
How is bioaccess® positioned to address clinical research challenges in Latin America?
Bioaccess® offers a complete range of services, including feasibility studies, site selection, compliance reviews, study setup, and project management, to enhance compliance and efficiency throughout the research study process.




